EXHIBIT 99.2
Published on November 9, 2022
Exhibit 99.2

INNOVATING FOR PATIENTS Nasdaq: TRVN I November 2022

Forward - Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts regarding Trevena, Inc. (t he “Company” or “we”), they are forward - looking statements reflecting management’s current beliefs and expectations. Forward - looking statements are subject to known and unknown risks, unc ertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward - looking statements by terminology such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “objective,” “predict,” “pr oject,” “suggest,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “ongoing,” or the negative of these terms or similar expressions. Forward - looking statements contained in this prese ntation include, but are not limited to, ( i ) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our pro duc t candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and ma rke t adoption of our potential drugs by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our cash needs. Actual results may differ materially from those indicated by such forward - looking statements as a result of various important f actors, including: the commercialization of any approved drug product, the status, timing, costs, results and interpretation of our clinical trials or any future trials of any of our inve sti gational drug candidates; the uncertainties inherent in conducting clinical trials; expectations for regulatory interactions, submissions and approvals, including our assessment of the discussions with th e FDA or other regulatory agencies about any and all of our programs; uncertainties related to the commercialization of OLINVYK; available funding; uncertainties related to our intellec tua l property; uncertainties related to the ongoing COVID - 19 pandemic, other matters that could affect the availability or commercial potential of our therapeutic candidates; and other f act ors discussed in the Risk Factors set forth in our Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q filed with the Securities and Exchange Commission (SEC) and in other filings we make with the SEC from time to time. In addition, the forward - looking statements included in this presentation represent our views only as of the date hereof. We anticipate that subs equent events and developments may cause our views to change. However, while we may elect to update these forward - looking statements at some point in the future, we specifically disc laim any obligation to do so, except as may be required by law. 2

BOARD OF DIRECTORS Leon O. Moulder , Jr. Chairman Marvin H. Johnson, Jr. Carrie L. Bourdow Jake R. Nunn Scott Braunstein, M.D. Anne M. Phillips, M.D. Michael R. Dougherty Barbara Yanni SENIOR MANAGEMENT Carrie L. Bourdow President & Chief Executive Officer Mark A. Demitrack , M.D . SVP, Chief Medical Officer Patricia Drake SVP, Chief Commercial Officer Barry Shin SVP, Chief Financial Officer Robert T. Yoder SVP, Chief Business Officer & Head of Commercial Operations Trevena’s Experienced Leadership Team 3
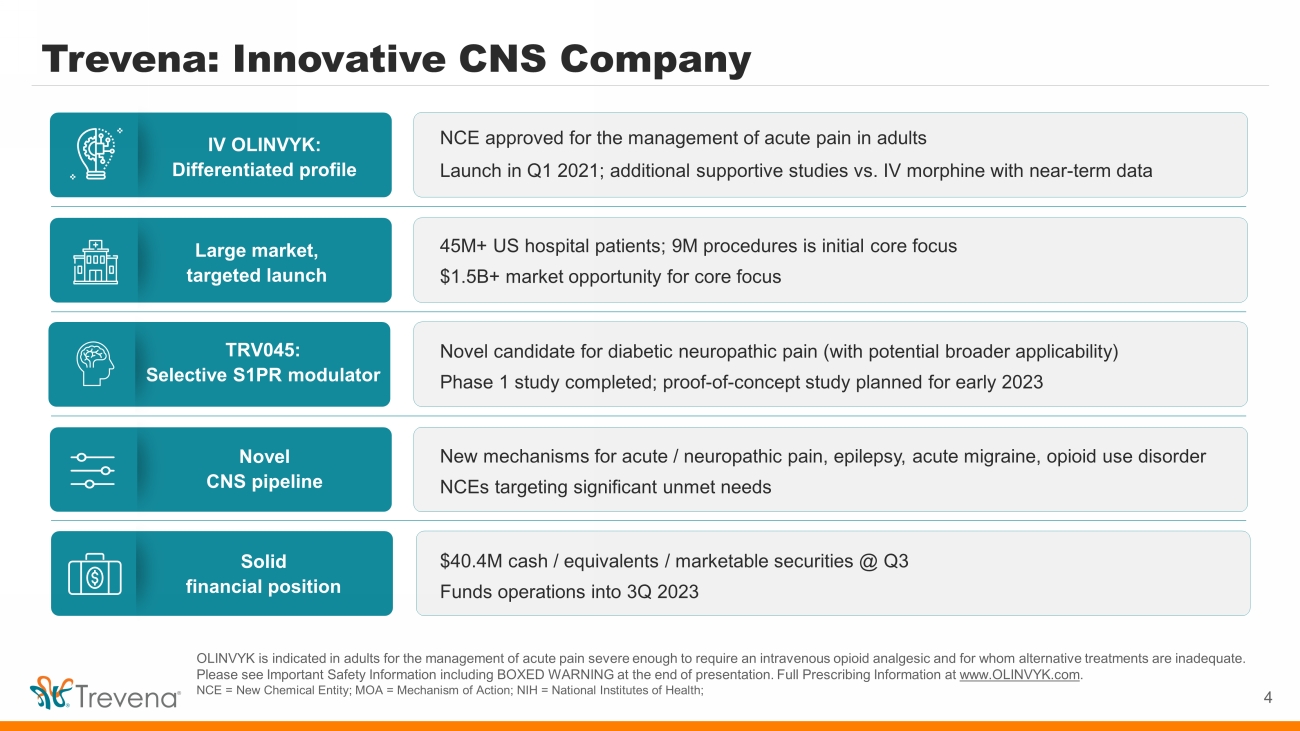
Trevena: Innovative CNS Company 4 OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . NCE = New Chemical Entity; MOA = Mechanism of Action; NIH = National Institutes of Health; IV OLINVYK: Differentiated profile Large market, targeted launch Novel CNS pipeline TRV045: Selective S1PR modulator Solid financial position NCE approved for the management of acute pain in adults Launch in Q1 2021; additional supportive studies vs. IV morphine with near - term data 45M+ US hospital patients; 9M procedures is initial core focus $1.5B+ market opportunity for core focus New mechanisms for acute / neuropathic pain, epilepsy, acute migraine, opioid use disorder NCEs targeting significant unmet needs Novel candidate for diabetic neuropathic pain (with potential broader applicability) Phase 1 study completed; proof - of - concept study planned for early 2023 $40.4M cash / equivalents / marketable securities @ Q3 Funds operations into 3Q 2023

PRE - CLIN PHASE 1 PHASE 2 PHASE 3 NDA POST - APPR EXPECTED CATALYSTS OLINVYK® New chemical entity (mu - opioid receptor) TRV045 Selective S1P receptor modulator • Phase 1 complete • Epilepsy data announced TRV250 G - protein selective agonist (delta receptor) • IND - enabling activities (oral) TRV734 G - protein selective agonist (mu - opioid receptor) Multiple Expected Catalysts 5 NIH / NIDA collab. IV OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. * Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at ww w.OLINVYK.com. TRV250, TRV734 and TRV045 are investigational products and are not approved by the FDA or any other regulatory agency.; NDA = Ne w Drug Application, PoC = Proof - of - Concept, DNP = Diabetic Neuropathic Pain IV acute pain* Respiratory physiology Clinical outcomes Cleveland Clinic / Wake Forest Baptist Health collab. Leiden UMC collab. DNP Acute migraine Epilepsy NIH collab. APPROVED • Topline data released March 2022 • Enrollment complete 2H 22 • Commercial launch ongoing • POC study ongoing Cognitive function • Topline data released July 2022 Opioid use disorder Nhwa NDA Submission in China • NDA Submitted Center for Human Drug Research, Leiden

Ex - US Royalty - Based Financing Highlights Blue Chip Investor R - Bridge Healthcare Fund affiliate of CBC Group (one of the largest and most active healthcare - dedicated investment firms in Asia) $40M Total Financing $15M upfront (received April 2022) $10M on commercial or financing milestone $15M on first commercial sale in China $40M total Flexible Payments* • Chinese Royalties. All royalties from Nhwa partnership, TRVN retains milestones • Capped US Royalty. 4% royalty on US OLINVYK net sales, with $10M cap** Constructive Terms • No financial covenants • Negative pledge only until Chinese approval • Flexibility for additional business development opportunities * R - Bridge will receive a 1.5% fee and warrants for 5M shares at a strike price of $0.82 / sh (75% premium to 30 - day VWAP) ** Potential increase to 7% (with combined US/China cap) if not approved by YE - 23 6
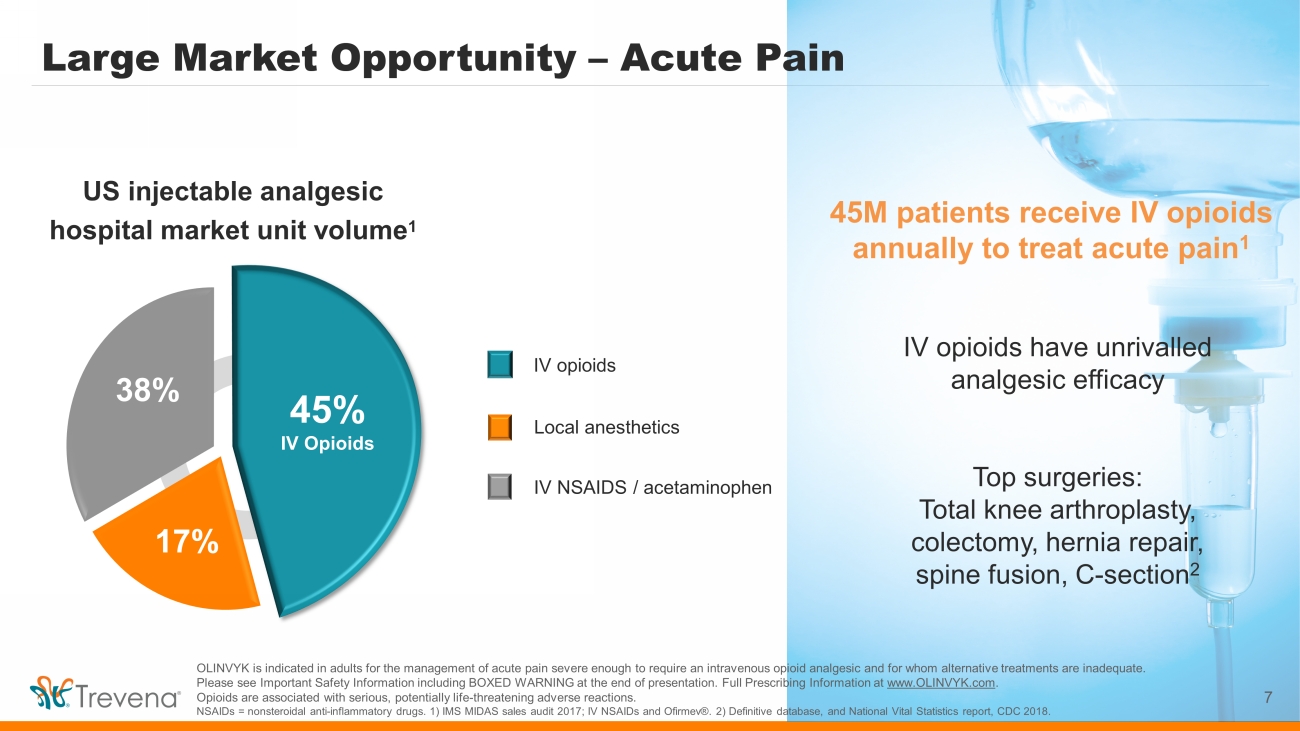
Large Market Opportunity – Acute Pain 7 45M patients receive IV opioids annually to treat acute pain 1 IV opioids have unrivalled analgesic efficacy Top surgeries: Total knee arthroplasty, colectomy, hernia repair, spine fusion, C - section 2 IV NSAIDS / acetaminophen US injectable analgesic hospital market unit volume 1 IV opioids 45% IV Opioids 17% 38% Local anesthetics OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . Opioids are associated with serious, potentially life - threatening adverse reactions. NSAIDs = nonsteroidal anti - inflammatory drugs. 1) IMS MIDAS sales audit 2017; IV NSAIDs and Ofirmev ®. 2) Definitive database, and National Vital Statistics report, CDC 2018.
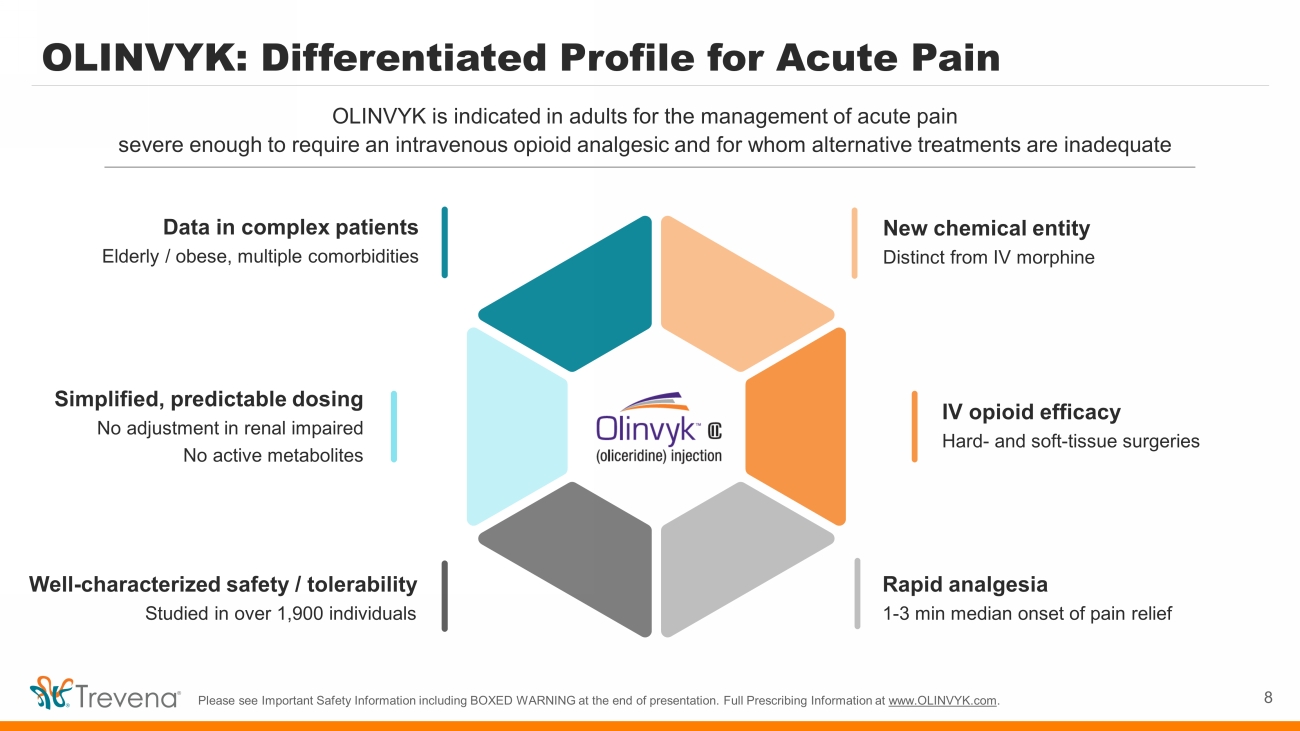
OLINVYK: Differentiated Profile for Acute Pain 8 OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . New chemical entity Distinct from IV morphine IV opioid efficacy Hard - and soft - tissue surgeries Rapid analgesia 1 - 3 min median onset of pain relief Simplified, predictable dosing No adjustment in renal impaired No active metabolites Data in complex patients Elderly / obese, multiple comorbidities Well - characterized safety / tolerability Studied in over 1,900 individuals
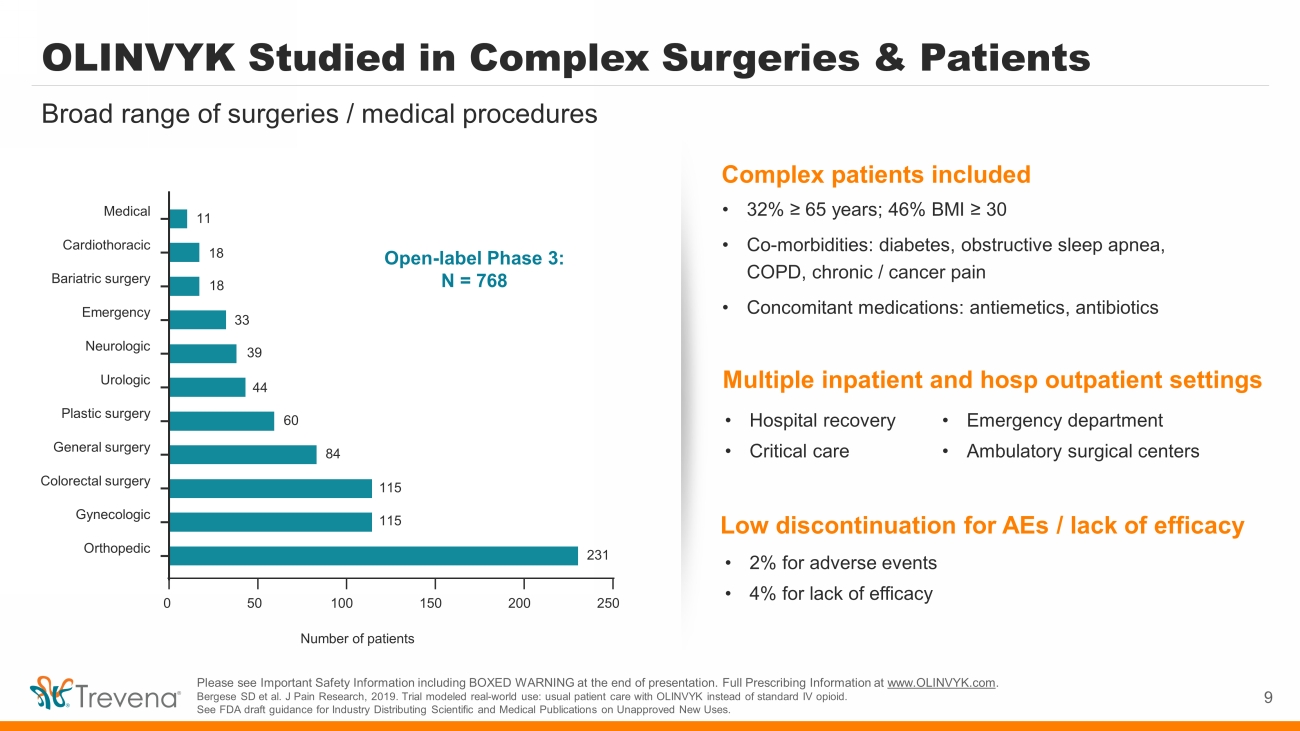
OLINVYK Studied in Complex Surgeries & Patients 9 Broad range of surgeries / medical procedures 0 50 100 150 200 250 Orthopedic Gynecologic Colorectal surgery General surgery Plastic surgery Urologic Neurologic Emergency Bariatric surgery Cardiothoracic Medical Number of patients 11 18 18 33 39 44 60 84 115 115 231 • 2% for adverse events • 4% for lack of efficacy • Hospital recovery • Critical care • Emergency department • Ambulatory surgical centers • 32% ≥ 65 years; 46% BMI ≥ 30 • Co - morbidities: diabetes, obstructive sleep apnea, COPD, chronic / cancer pain • Concomitant medications: antiemetics, antibiotics Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . Bergese SD et al. J Pain Research, 2019. Trial modeled real - world use: usual patient care with OLINVYK instead of standard IV opioid. See FDA draft guidance for Industry Distributing Scientific and Medical Publications on Unapproved New Uses. Open - label Phase 3: N = 768 Complex patients included Multiple inpatient and hosp outpatient settings Low discontinuation for AEs / lack of efficacy
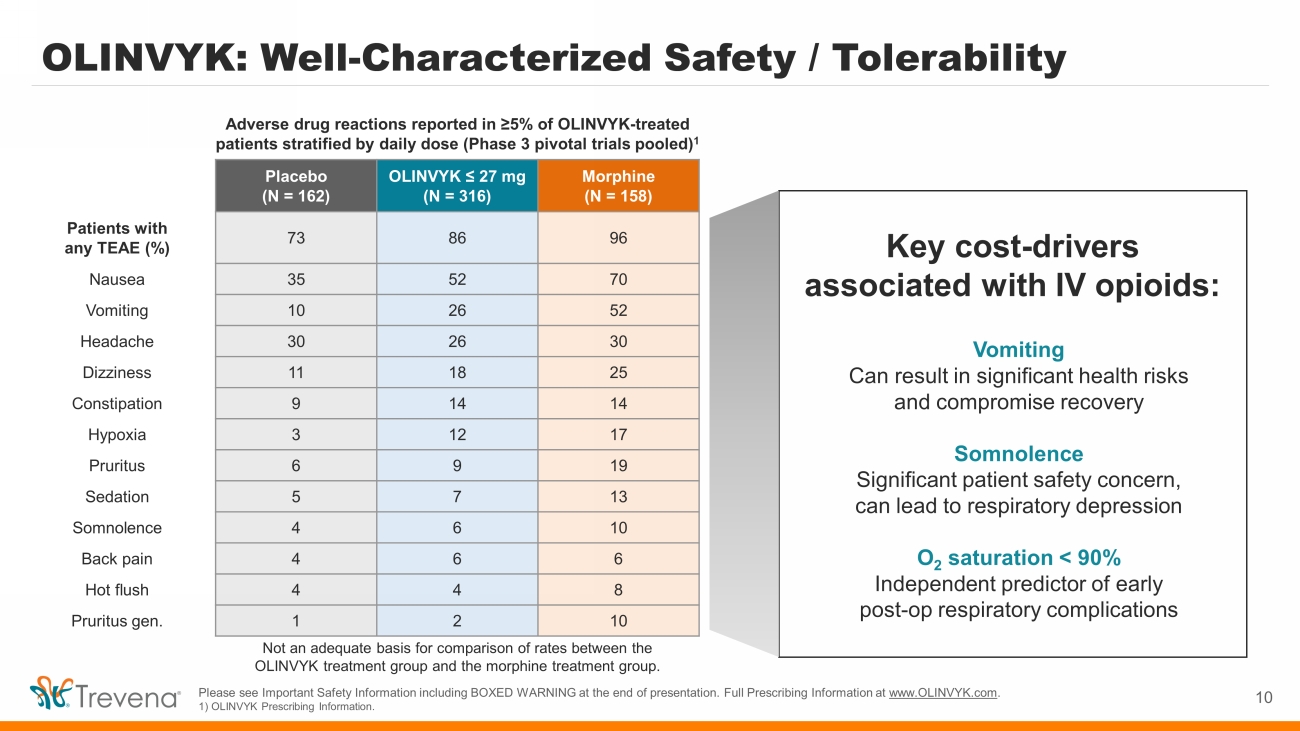
OLINVYK: Well - Characterized Safety / Tolerability 10 Placebo (N = 162) OLINVYK ≤ 27 mg (N = 316) Morphine (N = 158) Patients with any TEAE (%) 73 86 96 Nausea 35 52 70 Vomiting 10 26 52 Headache 30 26 30 Dizziness 11 18 25 Constipation 9 14 14 Hypoxia 3 12 17 Pruritus 6 9 19 Sedation 5 7 13 Somnolence 4 6 10 Back pain 4 6 6 Hot flush 4 4 8 Pruritus gen. 1 2 10 Adverse drug reactions reported in ≥5% of OLINVYK - treated patients stratified by daily dose (Phase 3 pivotal trials pooled) 1 Key cost - drivers associated with IV opioids: Vomiting Can result in significant health risks and compromise recovery Somnolence Significant patient safety concern, can lead to respiratory depression O 2 saturation < 90% Independent predictor of early post - op respiratory complications Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . 1) OLINVYK Prescribing Information. Not an adequate basis for comparison of rates between the OLINVYK treatment group and the morphine treatment group.
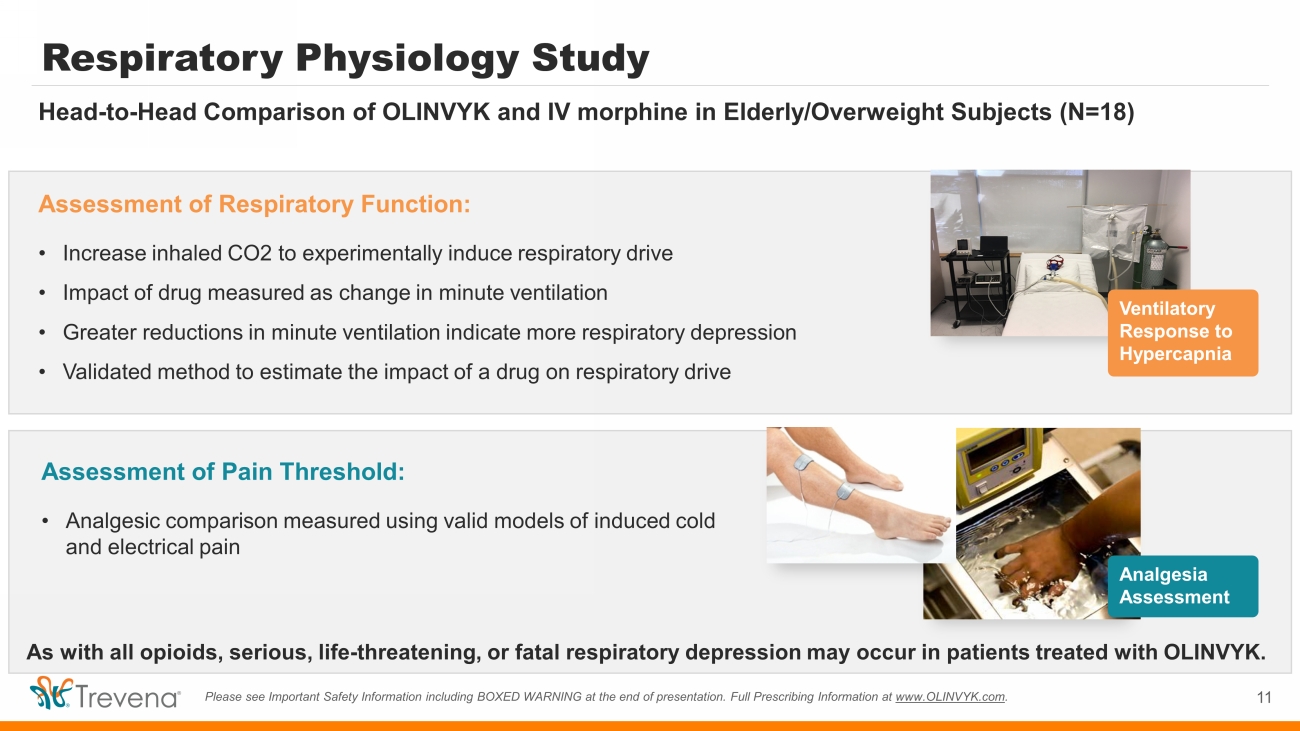
Respiratory Physiology Study Assessment of Respiratory Function: • Increase inhaled CO2 to experimentally induce respiratory drive • Impact of drug measured as change in minute ventilation • Greater reductions in minute ventilation indicate more respiratory depression • Validated method to estimate the impact of a drug on respiratory drive 11 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . Assessment of Pain Threshold: • Analgesic comparison measured using valid models of induced cold and electrical pain Ventilatory Response to Hypercapnia Analgesia Assessment As with all opioids, serious, life - threatening, or fatal respiratory depression may occur in patients treated with OLINVYK. Head - to - Head Comparison of OLINVYK and IV morphine in Elderly/Overweight Subjects (N=18)
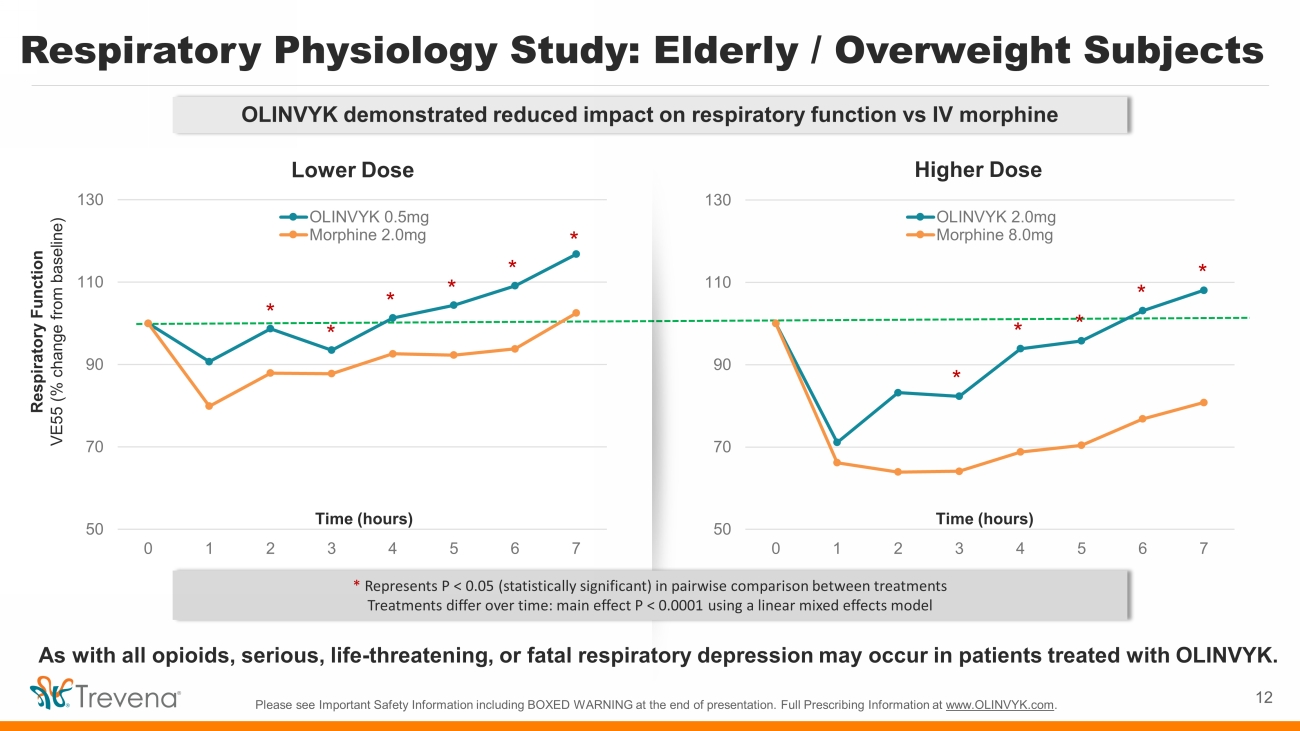
Respiratory Physiology Study: Elderly / Overweight Subjects Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . 12 50 70 90 110 130 0 1 2 3 4 5 6 7 OLINVYK 0.5mg Morphine 2.0mg 50 70 90 110 130 0 1 2 3 4 5 6 7 OLINVYK 2.0mg Morphine 8.0mg Respiratory Function VE55 (% change from baseline) Time (hours) * Represents P < 0.05 (statistically significant) in pairwise comparison between treatments T reatments differ over time: main effect P < 0.0001 using a linear mixed effects model * Time (hours) * * * * * * * * * * Lower Dose Higher Dose OLINVYK demonstrated reduced impact on respiratory function vs IV morphine As with all opioids, serious, life - threatening, or fatal respiratory depression may occur in patients treated with OLINVYK.

Respiratory Physiology Study Observations • Study population comprised elderly individuals (56 to 87 years, mean = 71.2) with BMI ranging from 20 to 34 (mean = 26.3) • Both OLINVYK and IV morphine achieved comparable levels of pain relief. A statistically significant reduced impact on respiratory function was observed in patients treated with OLINVYK as measured by the mean respiratory ventilation profiles over time (P<0.0001) • The study replicates the results from the earlier study in younger subjects using a similar methodology 1 . The findings extend our knowledge to patients who are at higher risk for the development of respiratory depression with the use of opioids, namely the elderly and overweight patients 13 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . As with all opioids, serious, life - threatening, or fatal respiratory depression may occur in patients treated with OLINVYK. 1. Soergel DG, et al. Pain. 2014;155:1829 - 1835
Top Line Data: OLINVYK vs IV Morphine Cognitive Function Study 14 Clinical assessment of OLINVYK’s potential impact on cognitive function vs. IV morphine • Randomized, double - blind, placebo - controlled, crossover study • N = 23 subjects, 19 - 53 years old (median age 26), 13 females & 10 males • Topline data received July 2022 Cognitive function assessment: NeuroCart • Comprehensive CNS test battery, used in testing a wide range of CNS drugs for 30 years • Cognitive outcome measures include major domains of motor performance, attention, reaction time, memory, and executive function Study will also include pain model testing (cold pressor test) and PK assessment Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .
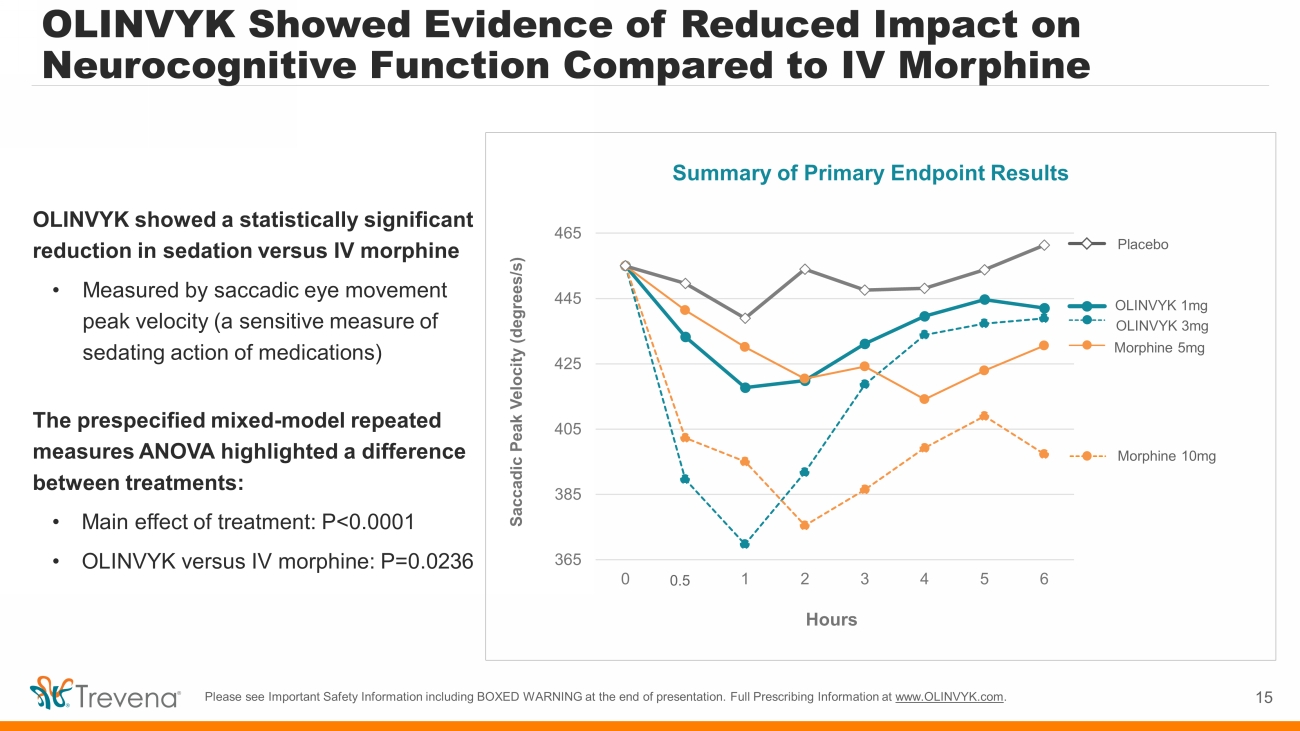
OLINVYK Showed Evidence of Reduced Impact on Neurocognitive Function Compared to IV Morphine 15 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . OLINVYK showed a statistically significant reduction in sedation versus IV morphine • Measured by saccadic eye movement peak velocity (a sensitive measure of sedating action of medications) The prespecified mixed - model repeated measures ANOVA highlighted a difference between treatments: • Main effect of treatment: P<0.0001 • OLINVYK versus IV morphine: P=0.0236 Summary of Primary Endpoint Results 365 385 405 425 445 465 0 0 1 2 3 4 5 6 Saccadic Peak Velocity (degrees/s) Hours Placebo Morphine 10mg OLINVYK 1mg OLINVYK 3mg Morphine 5mg 0.3 0.5
Secondary Endpoint Results • Reaction Time. Reduced impact on saccadic eye movement reaction time - M ain effect, P=0.0201 OLINVYK vs IV morphine, P=0.0273 • Postural Stability (Motor Function). Reduced body sway, a measure of motor function - M ain effect, P=0.0314 OLINVYK vs IV morphine, P=0.0951 • Eye - Hand Coordination . Reduced performance accuracy on the adaptive tracking test, a measure of eye - hand coordination - Main effect, P=0.0011 OLINVYK vs IV morphine, P=0.1303 • Neurocognitive function including impaired sedation and postural instability may have potentially important consequences in c lin ical care settings with the use of opioid medications, and consequent benefits in length of stay and other health economic outcome s • Other secondary outcome measures, including visual tracking and higher - order cognitive processing did not show statistical differences between OLINVYK and IV morphine • No serious adverse events were observed in the study, and adverse events were generally assessed as mild 16 OLINVYK showed a statistically significant difference or trend (vs IV morphine) on several prespecified secondary endpoints, despite the relatively small sample size, across a range of neurocognitive measures and motor performance: Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .
OLINVYK Safety Outcomes Study w/ Cleveland Clinic • Open - label, multi - site study led by experts at Cleveland Clinic and Wake Forest Baptist Health • N = ~200 adults undergoing major non - cardiac surgery • Enrollment complete 2H 2022 17 Further characterizes potential respiratory, GI and cognitive outcomes Respiratory Safety GI Tolerability Cognitive Function Predefined capnography and oximetry measures Assessment via continuous respiratory monitoring Complete GI response endpoint No vomiting and no antiemetic use through study period Somnolence, delirium, and sedation Validated, standardized assessment scales Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . As with all opioids, serious, life - threatening, or fatal respiratory depression may occur in patients treated with OLINVYK
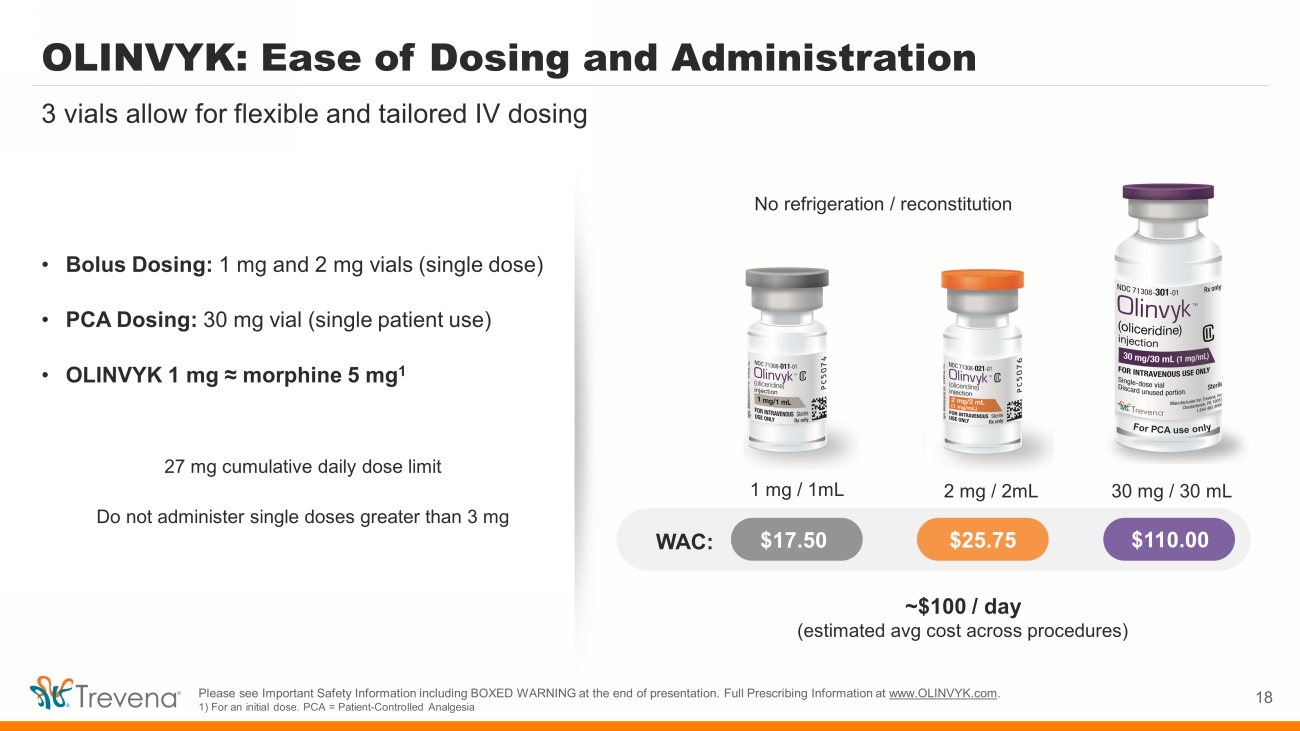
OLINVYK: Ease of Dosing and Administration • Bolus Dosing: 1 mg and 2 mg vials (single dose) • PCA Dosing: 30 mg vial (single patient use) • OLINVYK 1 mg ≈ morphine 5 mg 1 27 mg cumulative daily dose limit Do not administer single doses greater than 3 mg 18 3 vials allow for flexible and tailored IV dosing No refrigeration / reconstitution $17.50 $25.75 $110.00 WAC: 1 mg / 1mL 30 mg / 30 mL 2 mg / 2mL ~$100 / day (estimated avg cost across procedures) Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . 1) For an initial dose. PCA = Patient - Controlled Analgesia

OLINVYK vs IV Morphine Health Economic Models 19 Published 1 and available to formulary committees * As stated in the lable , these data are not an adequate basis for comparison of rates between OLINVYK treatment group and the morphine treatment gro up. The OLINVYK and morphine dosing regimens studied are not considered equipotent. 1) Simpson KN, et al., J Comp Eff Res, 2021 ; 10:1107 - 1119 and Simpson KN, et al. Expert Rev Pharmacoecon Outcomes Res; 2022 2) Oderda , GM, J Pain Palliative Care Pharm, 2019; data based on 5 surgical procedure categories including Cardiothoracic / vascular, Gen eral / Colorectal, Ob / Gyn, Orthopedic, and Urologic. 3) Overdyk FJ, PLoS One, 2016. More conservative inputs were used in the model. 4) Calculated based on total costs of Tx and average total costs of care. Image: flaticon.com. Vomiting Somnolence / sedation O 2 saturation <90% Representative Inputs: >10x Cost savings for hospitals 4 Due to improved patient outcomes HECON model Placebo (N = 162) OLINVYK ≤ 27 mg (N = 316) Morphine (N = 158) Patients with any TEAE (%) 73 86 96 Nausea 35 52 70 Vomiting 10 26 52 Headache 30 26 30 Dizziness 11 18 25 Constipation 9 14 14 Hypoxia 3 12 17 Pruritus 6 9 19 Sedation 5 7 13 Somnolence 4 6 10 Back pain 4 6 6 Hot flush 4 4 8 Pruritus gen. 1 2 10 AE rates * Cost of AEs Drug cost Ph3 trials Gov’t sources / Publications $8k nausea / vomiting 2 $28k critical resp event 3 +7 days hospital stay 3 OLINVYK IV morphine Key Outputs:

Customer Engagement Strategy
Targeted Account Launch 21 Health Care Practitioners (HCPs) Anesthesiology, Colorectal, Critical Care physicians Targeted Accounts Over 50 % of IV opioid volume covered by customer facing team Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . OLINVYK: NCE, distinct from IV morphine 1 - 3 min onset & no active metabolites Safety data in complex patients / surgeries OLINVYK published safety data Published health economic / cost offset data
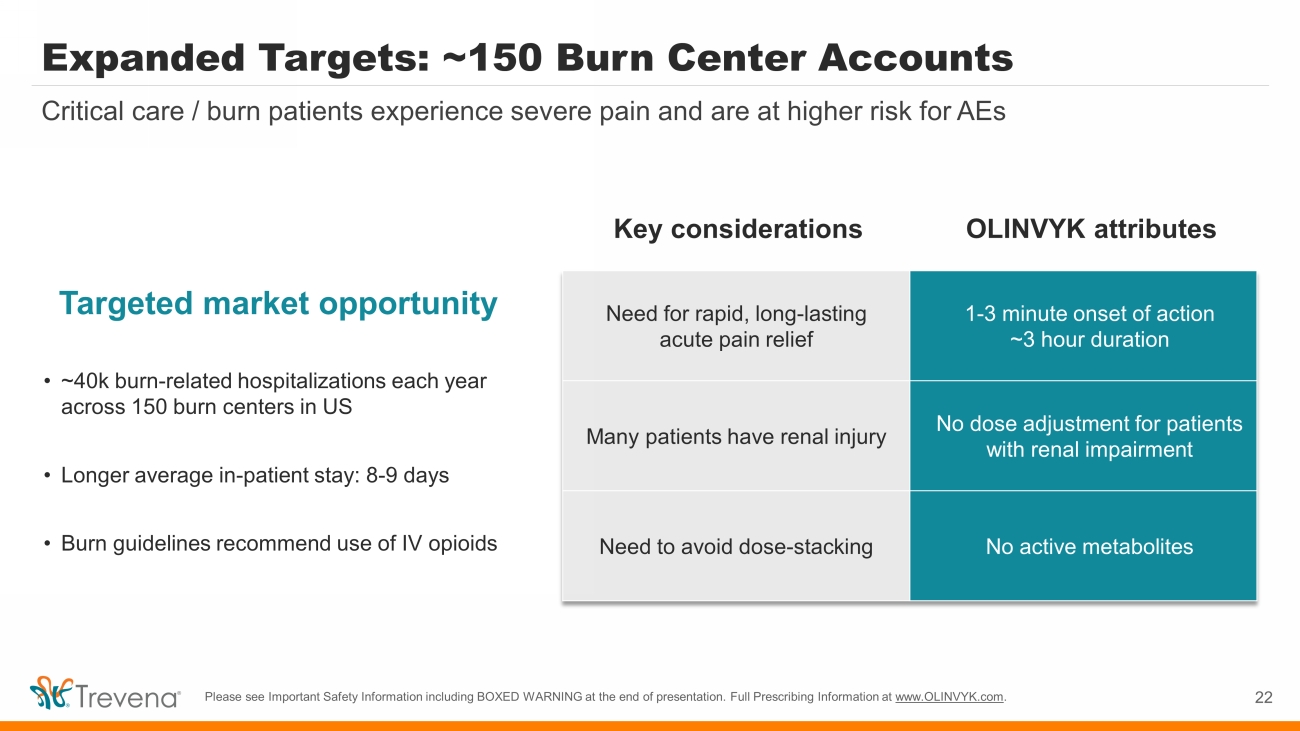
Expanded Targets: ~150 Burn Center Accounts Targeted market opportunity • ~40k burn - related hospitalizations each year across 150 burn centers in US • Longer average in - patient stay: 8 - 9 days • Burn guidelines recommend use of IV opioids 22 Critical care / burn patients experience severe pain and are at higher risk for AEs Need for rapid, long - lasting acute pain relief 1 - 3 minute onset of action ~3 hour duration Many patients have renal injury No dose adjustment for patients with renal impairment Need to avoid dose - stacking No active metabolites Key considerations OLINVYK attributes Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .

OLINVYK: Significant Opportunity in Acute Pain Market 23 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . Source: Definitive Healthcare; American Hospital Association. *Assumes ~$100 / day price for OLINVYK 2032 composition of matter patent expiration does not include potential patent extensions. Specialty Targets Patient & Procedure Risk Initial launch focus ~45 M patients Initial core focus: (9 M ) Expanded areas of focus (28 M ) • Hospitals / ambulatory surgical centers • Burn (6 - 8 days) / critical c are & c olorectal (3 - 5 days) Initial core focus ~15M days of therapy (initial focus) = $1.5B+ market opportunity* • New cognitive function / respiratory / GI data versus IV morphine • Additional HECON data focused on recovery time Expanded areas of focus 2032+ COM Patent

TRV045 S1P Receptor Modulator Novel MOA for Diabetic Neuropathic Pain
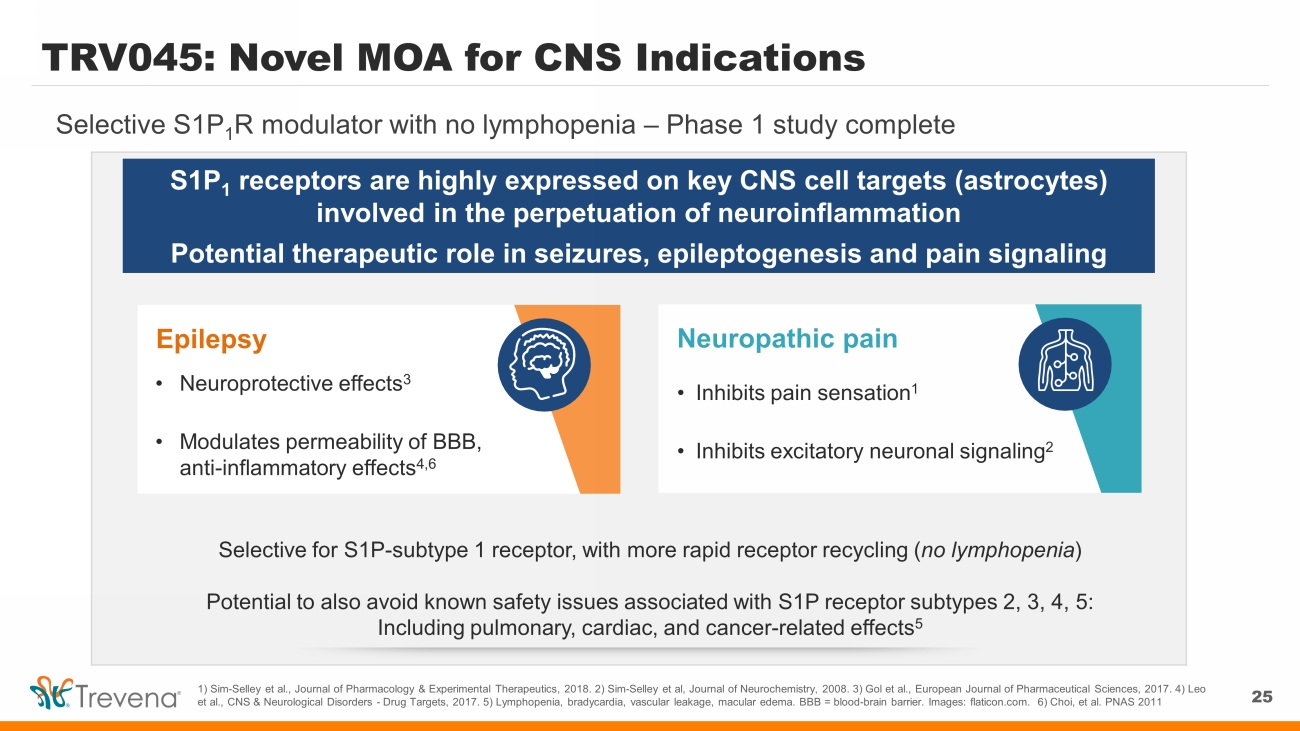
TRV045: Novel MOA for CNS Indications 25 Selective S1P 1 R modulator with no lymphopenia – Phase 1 study complete 1) Sim - Selley et al., Journal of Pharmacology & Experimental Therapeutics, 2018. 2) Sim - Selley et al, Journal of Neurochemistry, 2008. 3) Gol et al., European Journal of Pharmaceutical Sciences, 2017. 4) Leo et al., CNS & Neurological Disorders - Drug Targets, 2017. 5) Lymphopenia, bradycardia, vascular leakage, macular edema. BBB = b lood - brain barrier. Images: flaticon.com. 6) Choi, et al. PNAS 2011 S1P 1 receptors are highly expressed on key CNS cell targets (astrocytes) involved in the perpetuation of neuroinflammation Potential therapeutic role in seizures, epileptogenesis and pain signaling Neuropathic pain • Inhibits pain sensation 1 • Inhibits excitatory neuronal signaling 2 Selective for S1P - subtype 1 receptor, with more rapid receptor recycling ( no lymphopenia ) Potential to also avoid known safety issues associated with S1P receptor subtypes 2, 3, 4, 5: Including pulmonary, cardiac, and cancer - related effects 5 Epilepsy • Neuroprotective effects 3 • Modulates permeability of BBB, anti - inflammatory effects 4,6
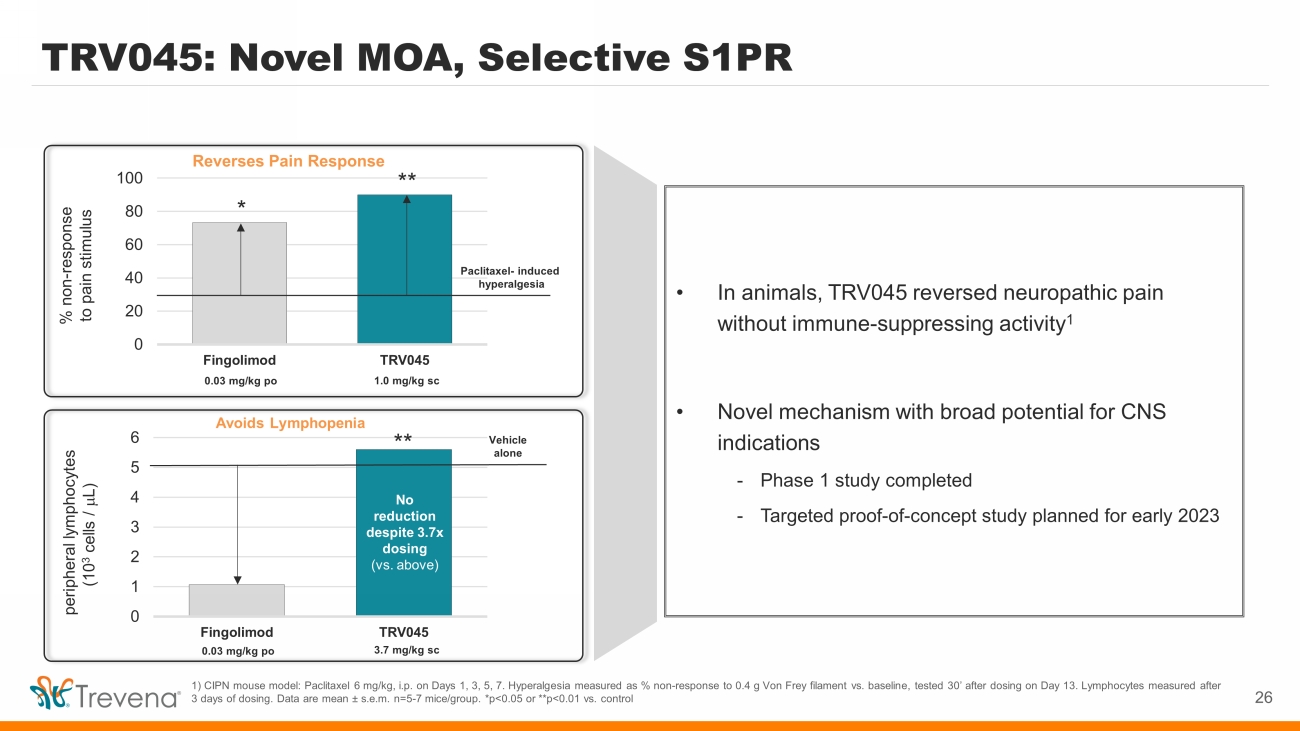
Avoids Lymphopenia TRV045: Novel MOA, Selective S1PR 26 1) CIPN mouse model: Paclitaxel 6 mg/kg, i.p. on Days 1, 3, 5, 7. Hyperalgesia measured as % non - response to 0.4 g Von Frey filament vs. baseline, tested 30’ after dosing on Day 13. Lymphocytes measured after 3 days of dosing. Data are mean s.e.m. n=5 - 7 mice/group. *p<0.05 or **p<0.01 vs. control • In animals, TRV045 reversed neuropathic pain without immune - suppressing activity 1 • Novel mechanism with broad potential for CNS indications - Phase 1 study completed - Targeted proof - of - concept study planned for early 2023 0 1 2 3 4 5 6 Fingolimod TRV045 peripheral lymphocytes (10 3 cells / m L) 0.03 mg/kg po 3.7 mg/kg sc Vehicle alone No reduction despite 3.7x dosing (vs. above) ** Reverses Pain Response 0 20 40 60 80 100 Fingolimod TRV045 % non - response to pain stimulus 0.03 mg/kg po 1.0 mg/kg sc Paclitaxel - induced hyperalgesia * **
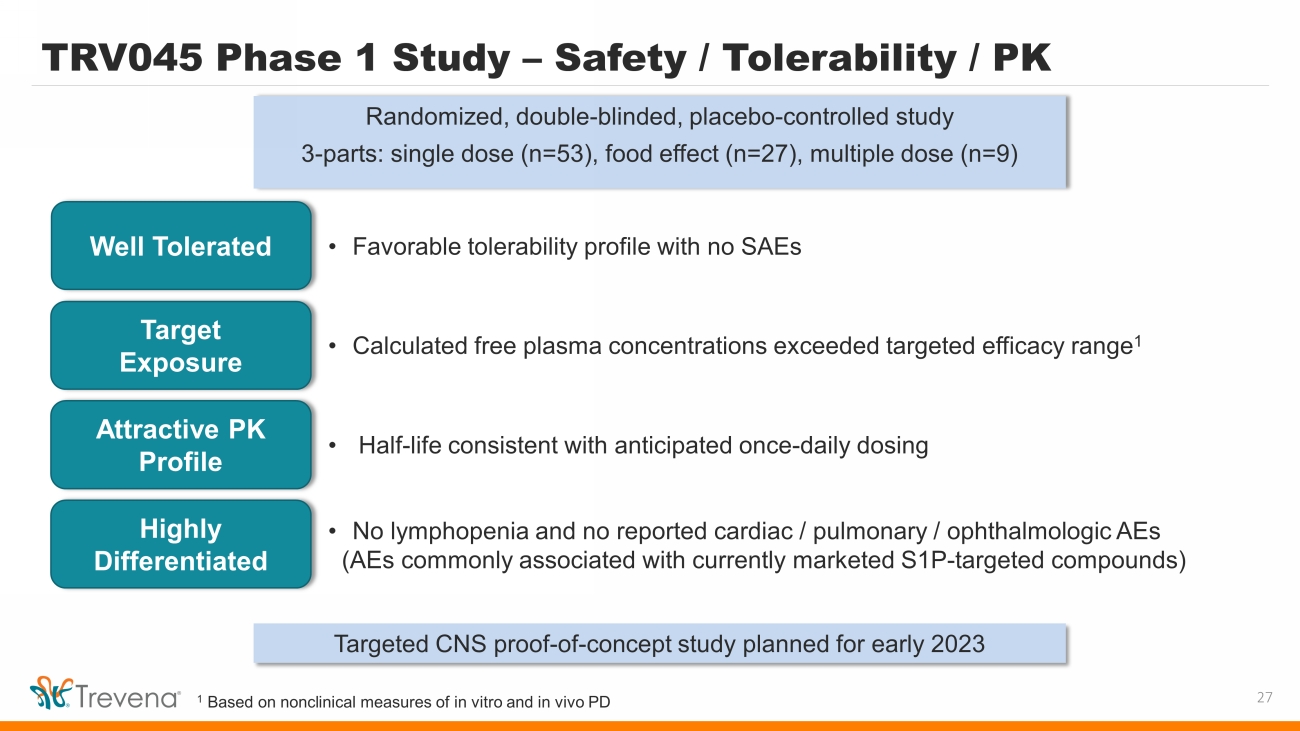
TRV045 Phase 1 Study – Safety / Tolerability / PK Randomized, double - blinded, placebo - controlled study 3 - parts: single dose (n=53), food effect (n=27), multiple dose (n=9) 27 • Favorable tolerability profile with no SAEs Well Tolerated Target Exposure Attractive PK Profile • Calculated free plasma concentrations exceeded targeted efficacy range 1 • Half - life consistent with anticipated once - daily dosing Targeted CNS proof - of - concept study planned for early 2023 Highly Differentiated • No lymphopenia and no reported cardiac / pulmonary / ophthalmologic AEs (AEs commonly associated with currently marketed S1P - targeted compounds) 1 Based on nonclinical measures of in vitro and in vivo PD

Effect of TRV045 on Cytokine / Chemokine Release • Methods: • Primary mouse astrocytes in monolayer cell culture; incubated for 24 hrs w/ 5 m M TRV045 • Panel of 17 cytokines / chemokines * assessed by ELISA • Main Findings: • Net anti - inflammatory action on astrocyte cytokine / chemokine release in culture • Increase in release of all anti - inflammatory cytokines measured (P<0.05 v vehicle) • Reduction in release of all inflammatory cytokines measured (P<0.05 v vehicle) 28 Anti - inflammatory actions on astrocytes in cell culture * Full cytokine / chemokine panel studied: (Inflammatory markers) – TNF a , IL - 6, IL - 1b, IL - 17, IL - 23, IL - 33, CCL1, CCL2, CCL20, CXCL5, CXCL12, CX3CL1, IFN g , Csf2, Substance P; (Anti - inflammatory markers) – IL - 10, IL - 4. [Trevena, Inc., data on file] -150 -100 -50 0 50 100 150 IL-1b TNFa IL-17 CXCL5 IL-4 IL-10 % Change from Vehicle Anti - inflammatory markers Inflammatory markers
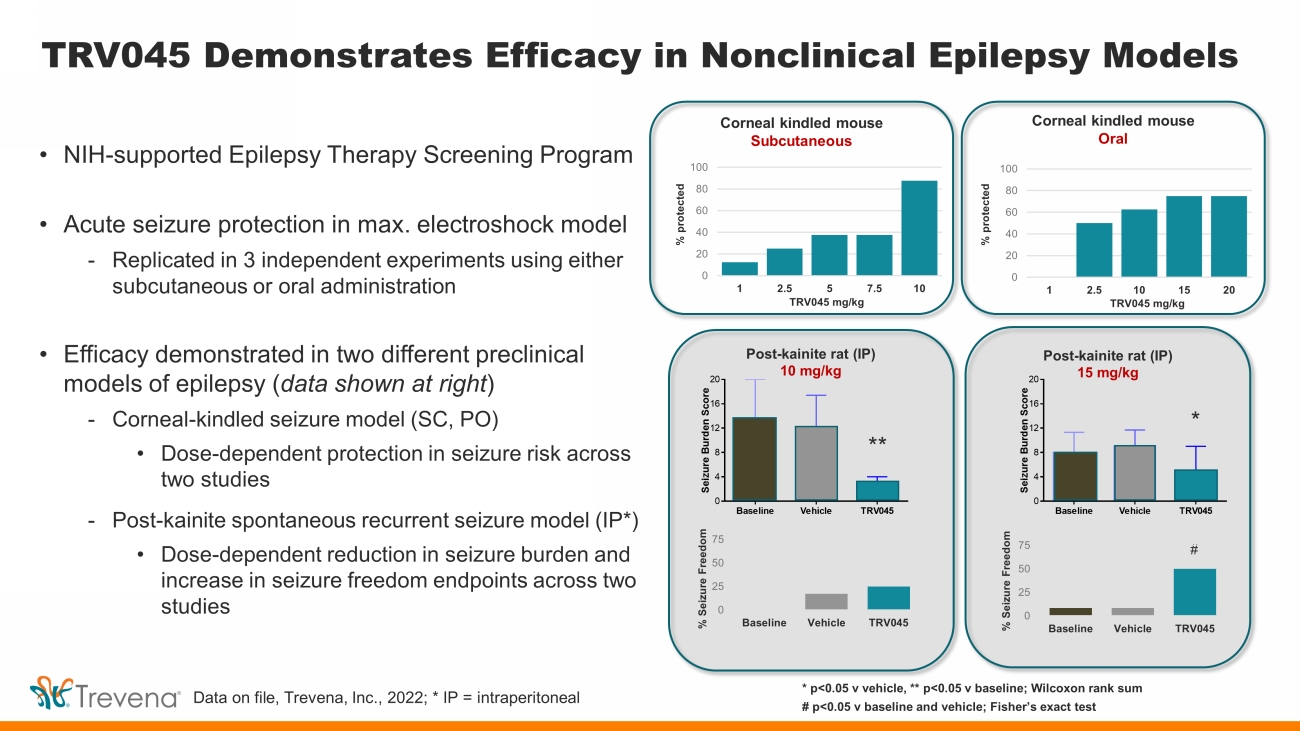
TRV045 Demonstrates Efficacy in Nonclinical Epilepsy Models • NIH - supported Epilepsy Therapy Screening Program • Acute seizure protection in max. electroshock model - Replicated in 3 independent experiments using either subcutaneous or oral administration • Efficacy demonstrated in two different preclinical models of epilepsy ( data shown at right ) - Corneal - kindled seizure model (SC, PO) • Dose - dependent protection in seizure risk across two studies - Post - kainite spontaneous recurrent seizure model (IP*) • Dose - dependent reduction in seizure burden and increase in seizure freedom endpoints across two studies Baseline Vehicle TRV045 0 4 8 12 16 20 S e i z u r e B u r d e n S c o r e * * p<0.05 v vehicle, ** p<0.05 v baseline; Wilcoxon rank sum # p<0.05 v baseline and vehicle; Fisher’s exact test Corneal kindled mouse Subcutaneous Corneal kindled mouse Oral Post - kainite rat (IP) 10 mg/kg Post - kainite rat (IP) 15 mg/kg 0 20 40 60 80 100 1 2.5 5 7.5 10 0 20 40 60 80 100 1 2.5 10 15 20 % protected % protected TRV045 mg/kg TRV045 mg/kg Data on file, Trevena, Inc., 2022; * IP = intraperitoneal ** * # 0 25 50 75 Baseline Vehicle TRV045 % Seizure Freedom 0 25 50 75 Baseline Vehicle TRV045 % Seizure Freedom

TRV250: New MOA for Acute Treatment of Migraine TRV734: Maintenance Therapy for Opioid Use Disorder

TRV250: New MOA for Acute Treatment of Migraine 31 Delta receptor: Untapped potential in CNS space Migraine represents a large market opportunity; total migraine drug market = ~$3.5B 1) Data from Decision Resources, Pharmacor migraine market landscape and forecast 2018. 2) Moven et al., J Neurol Neurosurg Psychiatry, 2016. Icons made by Freepik from www.flaticon.com Play important role in regulation of pain, mood, and anxiety Delta receptors have unique distribution throughout the brain 650M migraines treated each year 1.2M ER visits due to migraines • 20 - 30% of migraine sufferers do not respond to / cannot tolerate the market - leading triptan drug class • Approx. 50% of migraineurs also suffer from anxiety 2 Every year in the US 1 :
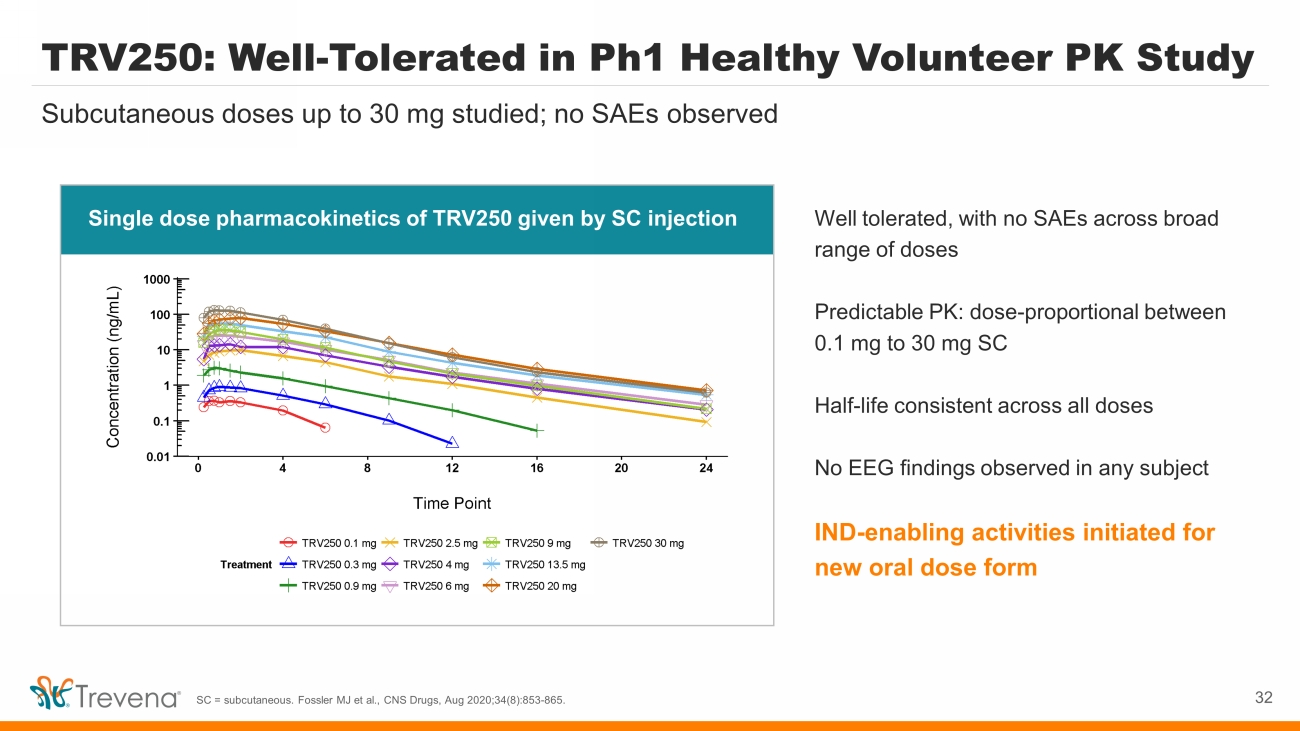
TRV250: Well - Tolerated in Ph1 Healthy Volunteer PK Study Well tolerated, with no SAEs across broad range of doses Predictable PK: dose - proportional between 0.1 mg to 30 mg SC Half - life consistent across all doses No EEG findings observed in any subject IND - enabling activities initiated for new oral dose form Subcutaneous doses up to 30 mg studied; no SAEs observed SC = subcutaneous. Fossler MJ et al., CNS Drugs, Aug 2020;34(8):853 - 865. 32 Single dose pharmacokinetics of TRV250 given by SC injection

TRV734: Maintenance Therapy for Opioid Use Disorder 33 Selective agonism at µ receptor: nonclinical evidence of improved tolerability 1) Center for Behavioral Health Statistics and Quality. 2) NIDA data on file. Ongoing collaboration with National Institute on Drug Abuse (NIDA) >2.5M people in U.S. suffer from opioid use disorder 1 NIDA study demonstrated reduced drug - seeking behavior in animal model of relapse 2 NIDA - funded proof - of - concept patient study initiated • Randomized, double - blind, placebo - and positive - controlled study • N = ~50 opioid - dependent patients undergoing stable methadone maintenance therapy • Primary endpoint: s uppression of withdrawal symptoms as measured by the Subjective Opioid Withdrawal Scale • Secondary outcomes: assessments of safety, tolerability, and neurocognitive changes

Trevena: Innovative CNS Company 34 OLINVYK is indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . NCE = New Chemical Entity; MOA = Mechanism of Action; NIH = National Institutes of Health; IV OLINVYK: Differentiated profile Large market, targeted launch Novel CNS pipeline TRV045: Selective S1PR modulator Solid financial position NCE approved for the management of acute pain in adults Launch in Q1 2021; additional supportive studies vs. IV morphine with near - term data 45M+ US hospital patients; 9M procedures is initial core focus $1.5B+ market opportunity for core focus New mechanisms for acute / neuropathic pain, epilepsy, acute migraine, opioid use disorder NCEs targeting significant unmet needs Novel candidate for diabetic neuropathic pain (with potential broader applicability) Phase 1 study completed; proof - of - concept study planned for early 2023 $40.4M cash / equivalents / marketable securities @ Q3 Funds operations into 3Q 2023

35 JL: can will create a transition slide? Appendix
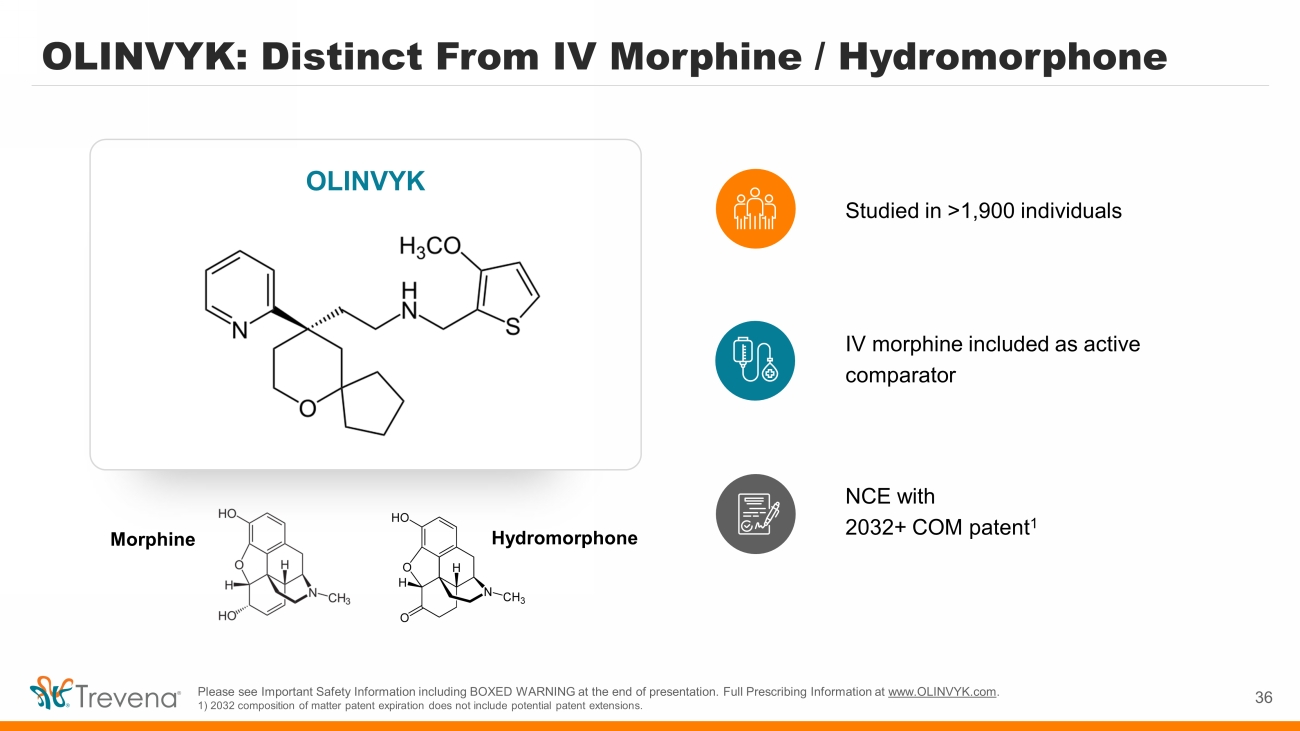
Studied in >1,900 individuals Hydromorphone OLINVYK: Distinct From IV Morphine / Hydromorphone Morphine 36 OLINVYK IV morphine included as active comparator NCE with 2032+ COM patent 1 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . 1) 2032 composition of matter patent expiration does not include potential patent extensions.
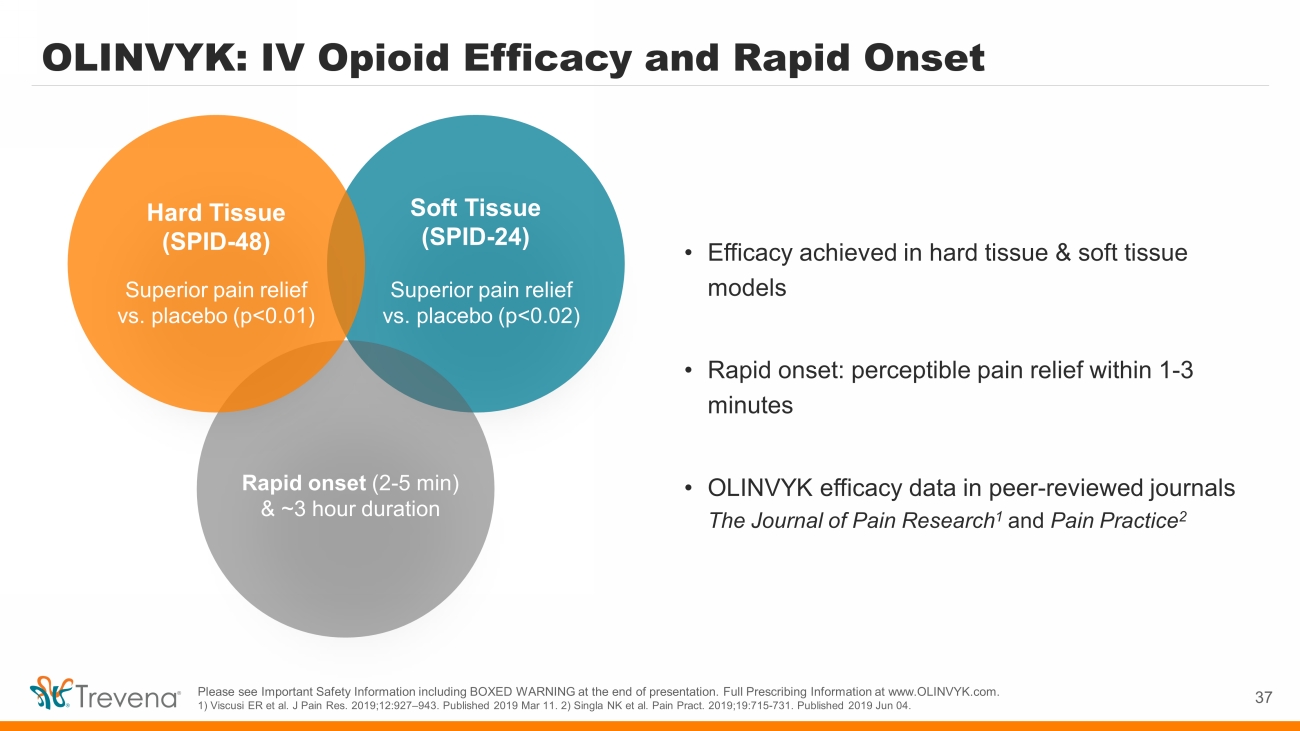
OLINVYK: IV Opioid Efficacy and Rapid Onset 37 • Efficacy achieved in hard tissue & soft tissue models • Rapid onset: perceptible pain relief within 1 - 3 minutes • OLINVYK efficacy data in peer - reviewed journals The Journal of Pain Research 1 and Pain Practice 2 Soft Tissue (SPID - 24) Rapid onset (2 - 5 min) & ~3 hour duration Superior pain relief vs. placebo (p<0.01) Hard Tissue (SPID - 48) Superior pain relief vs. placebo (p<0.02) Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at w ww. OLINVYK.com. 1) Viscusi ER et al. J Pain Res. 2019;12:927 – 943. Published 2019 Mar 11. 2) Singla NK et al. Pain Pract . 2019;19:715 - 731. Published 2019 Jun 04.
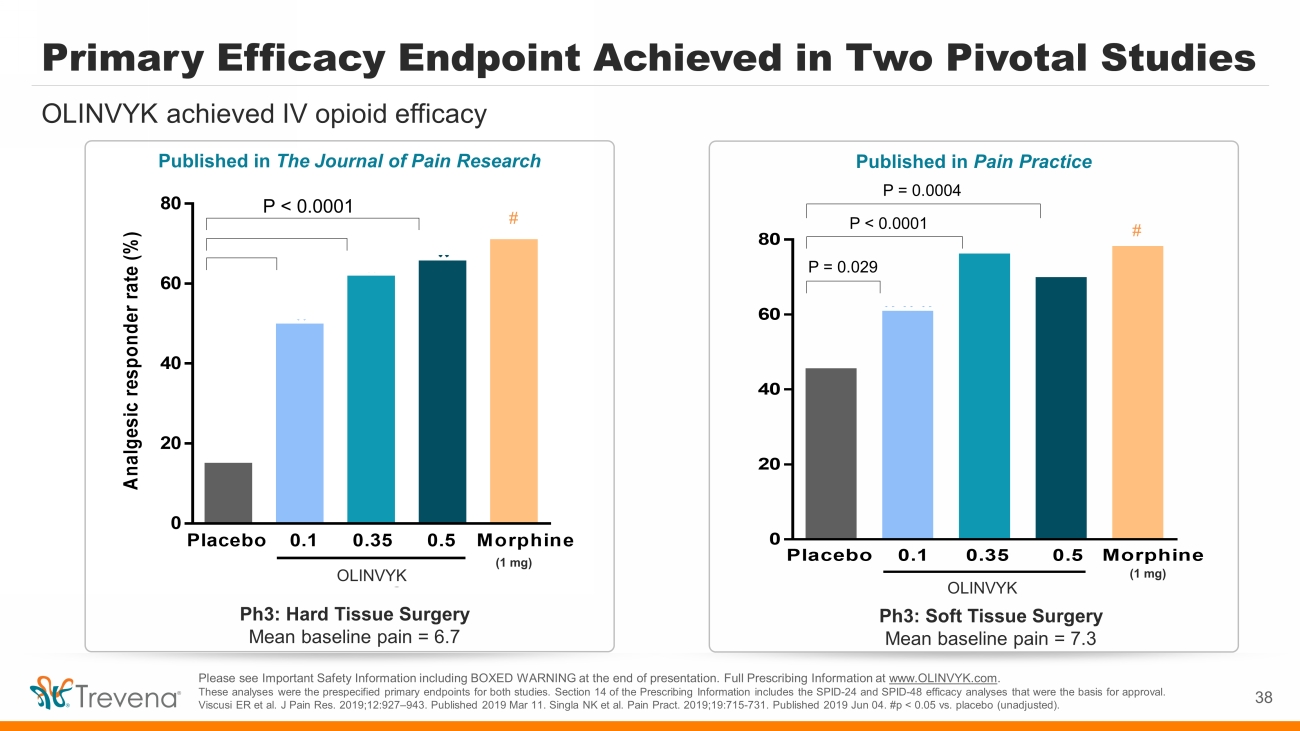
38 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . These analyses were the prespecified primary endpoints for both studies. Section 14 of the Prescribing Information includes t he SPID - 24 and SPID - 48 efficacy analyses that were the basis for approval. Viscusi ER et al. J Pain Res. 2019;12:927 – 943. Published 2019 Mar 11. Singla NK et al. Pain Pract . 2019;19:715 - 731. Published 2019 Jun 04. #p < 0.05 vs. placebo (unadjusted). 0 20 40 60 80 Placebo 0.1 0.35 0.5 Morphine *** Olinvo Regimen * ** # P = 0.029 P < 0.0001 P = 0.0004 OLINVYK Ph3: Soft Tissue Surgery Mean baseline pain = 7.3 (1 mg) 0 20 40 60 80 Placebo 0.1 0.35 0.5 Morphine * A n a l g e s i c r e s p o n d e r r a t e ( % ) Olinvo Regimen * * Ph3: Hard Tissue Surgery Mean baseline pain = 6.7 # P < 0.0001 OLINVYK (1 mg) Primary Efficacy Endpoint Achieved in Two Pivotal Studies OLINVYK achieved IV opioid efficacy Published in Pain Practice Published in The Journal of Pain Research

0 1 2 3 4 5 6 7 8 9 10 0 4 8 12 16 20 24 28 32 36 40 44 48 0 1 2 3 4 5 6 7 8 9 10 0 4 8 12 16 20 24 Time (hours) Average NRS Pain Score OLINVYK: IV Opioid Efficacy in 2 Phase 3 RCTs Study 1 (Orthopedic – Hard Tissue) 3 PCA regimens studied (0.1, 0.35, 0.5 mg) vs. placebo; all doses P<0.01 vs. placebo 39 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . OLINVYK Outcome 0.1 mg 0.35 mg 0.5 mg Placebo % Completed 83% 87% 84% 60% % D/C LOE 9% 4% 5% 34% % Rescue Meds 41% 20% 17% 77% Study 2 (Plastic Surgery – Soft Tissue) 3 PCA regimens studied (0.1, 0.35, 0.5 mg) vs. placebo; 0.35 / 0.5 mg doses P<0.02 vs. placebo OLINVYK Outcome 0.1 mg 0.35 mg 0.5 mg Placebo % Completed 86% 90% 87% 74% % D/C LOE 11% 3% 5% 22% % Rescue Meds 31% 21% 18% 49% Placebo (n=79) OLINVYK 0.1mg (n=76) OLINVYK 0.35mg (n=79) OLINVYK 0.5mg (n=79) Placebo (n=81) OLINVYK 0.1mg (n=77) OLINVYK 0.35mg (n=80) OLINVYK 0.5mg (n=80)
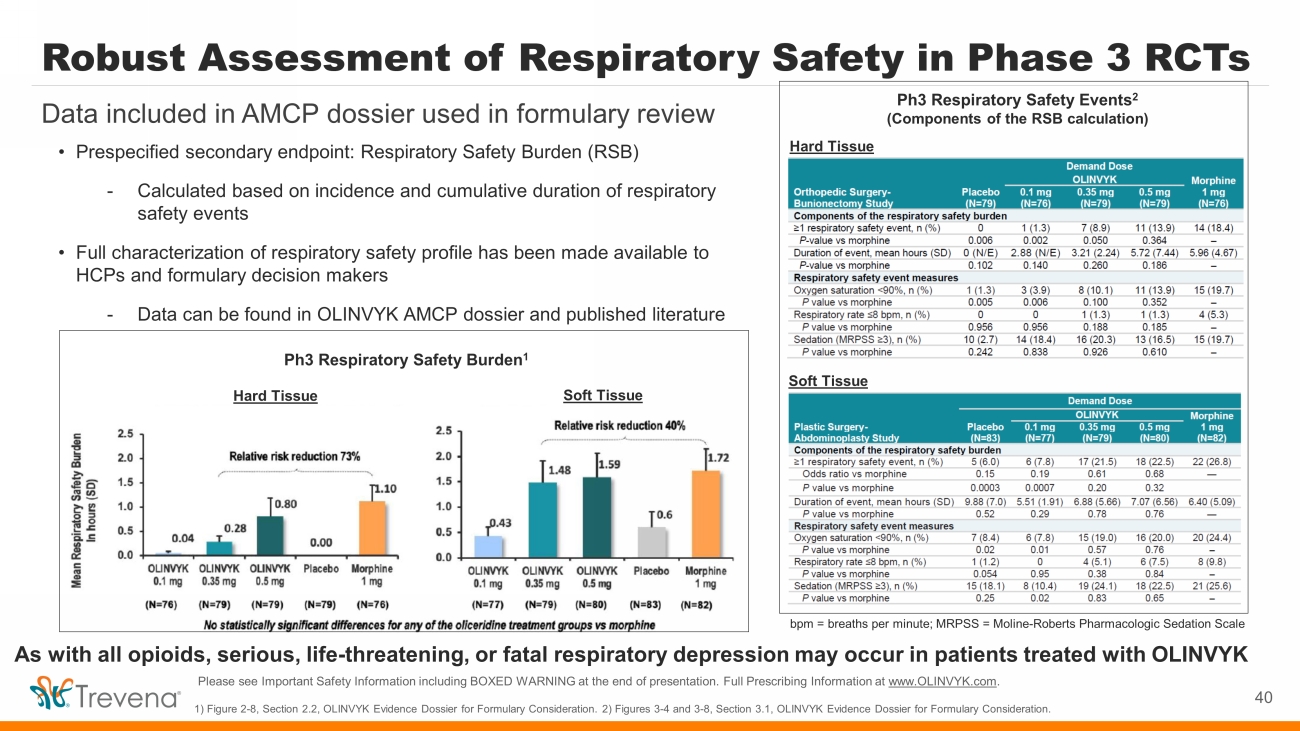
Robust Assessment of Respiratory Safety in Phase 3 RCTs 40 Data included in AMCP dossier used in formulary review 1) Figure 2 - 8, Section 2.2, OLINVYK Evidence Dossier for Formulary Consideration. 2) Figures 3 - 4 and 3 - 8, Section 3.1, OLINVYK E vidence Dossier for Formulary Consideration. • Prespecified secondary endpoint: Respiratory Safety Burden (RSB) - Calculated based on incidence and cumulative duration of respiratory safety events • Full characterization of respiratory safety profile has been made available to HCPs and formulary decision makers - Data can be found in OLINVYK AMCP dossier and published literature Ph3 Respiratory Safety Events 2 (Components of the RSB calculation) Hard Tissue Soft Tissue bpm = breaths per minute; MRPSS = Moline - Roberts Pharmacologic Sedation Scale Ph3 Respiratory Safety Burden 1 Hard Tissue Soft Tissue As with all opioids, serious, life - threatening, or fatal respiratory depression may occur in patients treated with OLINVYK Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .

Robust Assessment of GI Tolerability in Phase 3 RCTs 41 Data included in AMCP dossier used in formulary review P < 0.05 vs. morphine.1) Figure 2 - 10, Section 2.2, OLINVYK Evidence Dossier for Formulary Consideration. 2) Figure 2 - 11, Section 2.2, OLINVYK Evidence Dossier for Formulary Consideration. 3) GI complete response defined as the proportion of patients who did not experience the AE of vomiting and did not use rescue anti eme tic medication throughout their allocated treatment period in the study. Ph3 Rescue Antiemetic Use 1 Hard Tissue Soft Tissue Ph3 Complete GI Response Rates 2 Hard Tissue Soft Tissue • Phase 3 pivotal trials included measurements of nausea / vomiting rates and rescue antiemetic use • Additional exploratory post - hoc analysis was conducted using a “complete GI response” endpoint 3 • Full characterization of GI tolerability has been made available to HCPs and formulary decision makers - Data can be found in OLINVYK AMCP dossier and published literature Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .
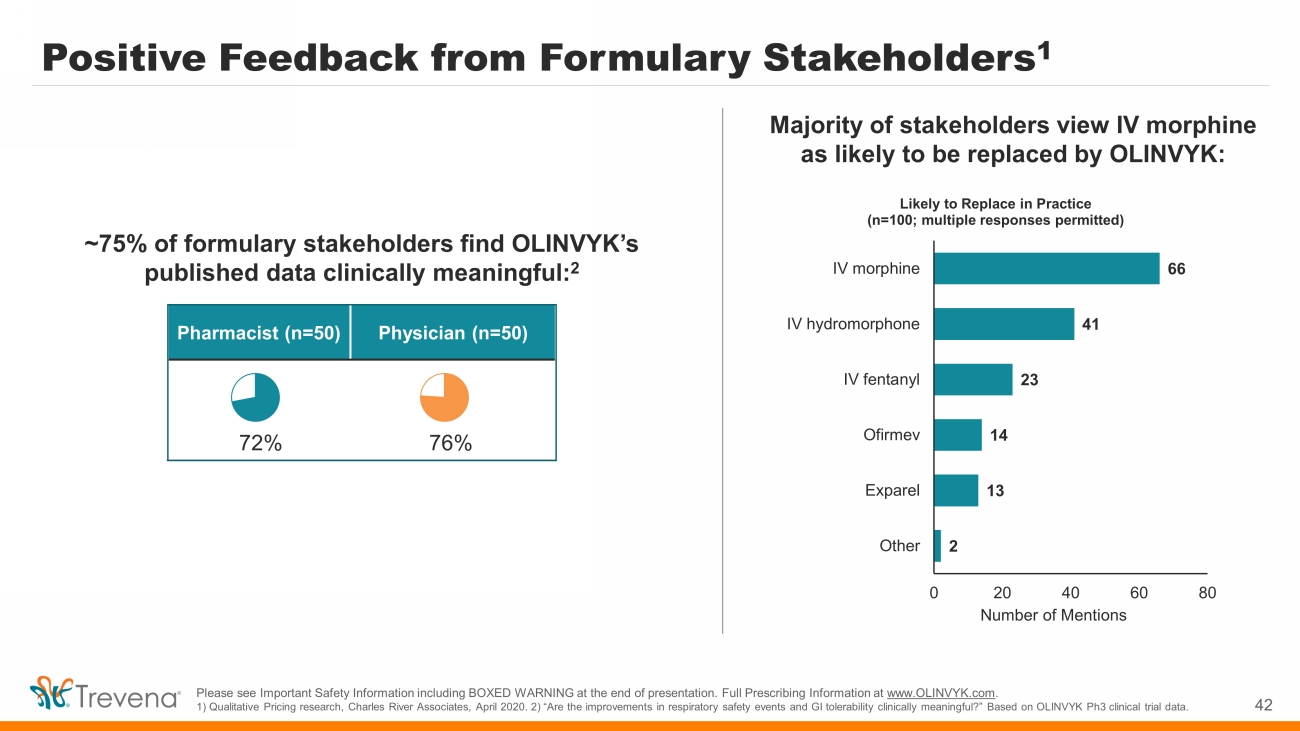
Positive Feedback from Formulary Stakeholders 1 42 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com . 1) Qualitative Pricing research, Charles River Associates, April 2020. 2) “Are the improvements in respiratory safety events and GI tolerability clinically meaningful?” Based on OLINVYK Ph3 clinical trial data. ~75% of formulary stakeholders find OLINVYK’s published data clinically meaningful: 2 66 41 23 14 13 2 0 20 40 60 80 IV morphine IV hydromorphone IV fentanyl Ofirmev Exparel Other Number of Mentions Likely to Replace in Practice (n=100; multiple responses permitted) Pharmacist (n=50) Physician (n=50) 72% 76% Majority of stakeholders view IV morphine as likely to be replaced by OLINVYK:

Omni - channel Approach for HCP Engagement 43 Communication across a full range of channels to maximize reach and impact Field directed: live, virtual & email HCP social media Professional Society Meetings & Congresses Olinvyk.com Virtual “on demand” Medical Education programs Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.OLINVYK.com .
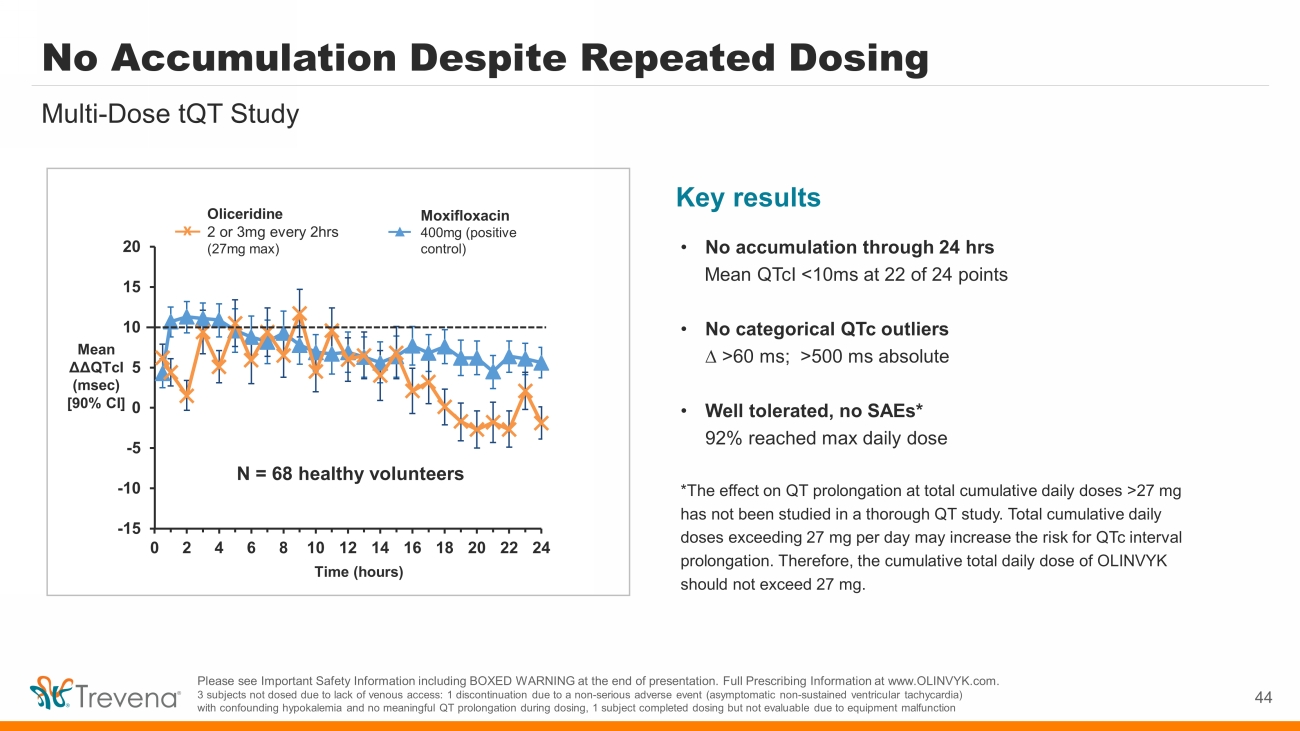
-15 -10 -5 0 5 10 15 20 0 2 4 6 8 10 12 14 16 18 20 22 24 Mean ΔΔ QTcI ( msec ) [90% CI] No Accumulation Despite Repeated Dosing 44 Multi - Dose tQT Study Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at w ww. OLINVYK.com. 3 subjects not dosed due to lack of venous access: 1 discontinuation due to a non - serious adverse event (asymptomatic non - sustai ned ventricular tachycardia) with confounding hypokalemia and no meaningful QT prolongation during dosing, 1 subject completed dosing but not evaluable du e t o equipment malfunction Oliceridine 2 or 3mg every 2hrs (27mg max) x Moxifloxacin 400mg (positive control) Time (hours) N = 68 healthy volunteers • No accumulation through 24 hrs Mean QTcI <10ms at 22 of 24 points • No categorical QTc outliers ∆ >60 ms ; >500 ms absolute • Well tolerated, no SAEs* 92% reached max daily dose *The effect on QT prolongation at total cumulative daily doses >27 mg has not been studied in a thorough QT study. Total cumulative daily doses exceeding 27 mg per day may increase the risk for QTc interval prolongation. Therefore, the cumulative total daily dose of OLINVYK should not exceed 27 mg. Key results
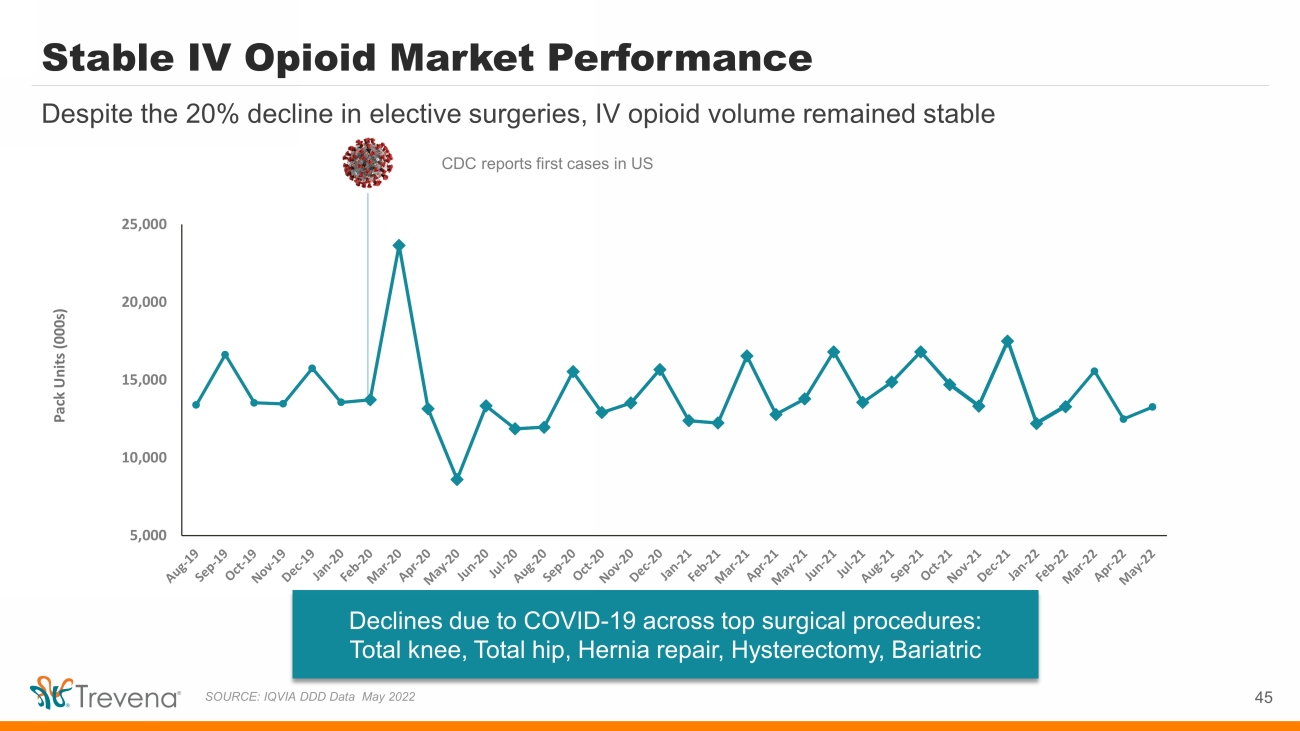
Stable IV Opioid Market Performance 45 Despite the 20% decline in elective surgeries, IV opioid volume remained stable SOURCE: IQVIA DDD Data May 2022 Declines due to COVID - 19 across top surgical procedures: Total knee, Total hip, Hernia repair, Hysterectomy, Bariatric Pack Units (000s) CDC reports first cases in US 5,000 10,000 15,000 20,000 25,000 Thousands
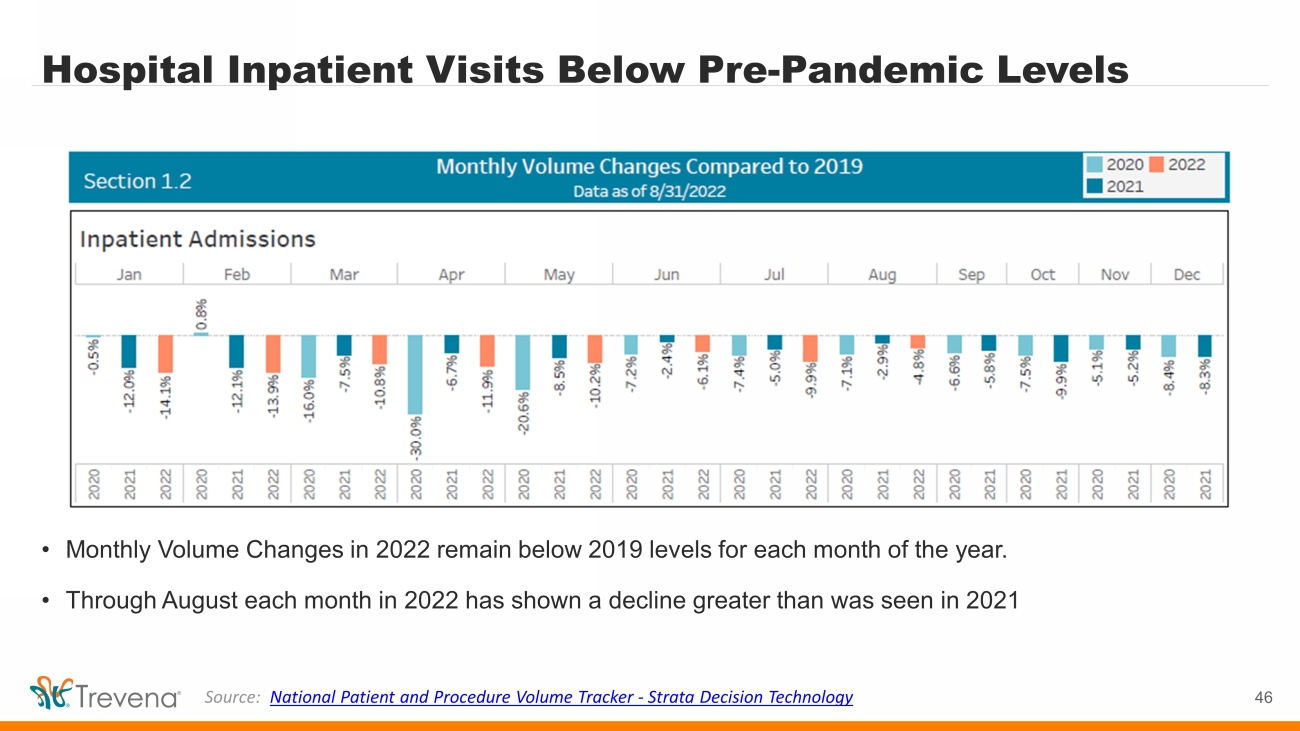
Hospital Inpatient Visits Below Pre - Pandemic Levels • Monthly Volume Changes in 2022 remain below 2019 levels for each month of the year. • Through August each month in 2022 has shown a decline greater than was seen in 2021 46 Source: National Patient and Procedure Volume Tracker - Strata Decision Technology

$0 $7,000 $14,000 $21,000 $28,000 $35,000 Diabetes only Diabetes with DNP Diabetic Neuropathic Pain 47 Diabetic neuropathic pain (DNP) represents a large market opportunity 1) IDF, www.diabetesatlas.org 2) Economic Costs of Diabetes in the U.S. in 2017, Diabetes Care 2018;41:917 – 928. 3) Shillo et al., Current Diabetes Reports, 2019 4) Pritchett, YL et al. Pain Medicine 2007 5) Freeman R et al., Diabetes Care 2008 6) Sadosky et. al., J Diabetes Complications 2015. 7) Datamonitor 8) Hicks, et al. Current Diabetes Reports, 2019 • 30M+ US adults with diabetes (500M+ worldwide) 1,2 • DNP affects up to 25% of patients with diabetes 3,8 • Significant need for efficacious medicines for DNP 4 - 5 » Only ~50% of patients experience a clinical response with currently approved therapies • Direct costs for patients with DNP were ~4x that of patients with only diabetes (no DNP) 6 Annual Direct Costs (per patient)

Epilepsy 48 One of the most common neurological diseases in the world 1 1. World Health Organization. Epilepsy. https://www.who.int/news - room/fact - sheets/detail/epilepsy. Accessed November, 2021. 2. Epi lepsy Foundation. About Epilepsy: The Basics. https://www.epilepsy.com/learn/about - epilepsy - basics. Accessed November, 2021. 3. Epilepsy Foundation. What is a Seizure? https ://www.epilepsy.com/learn/about - epilepsy - basics/what - seizure. Accessed November, 2021.4. CURE Epilepsy. What is epilepsy? https://www.cureepilepsy.org/about - epilepsy/what - is - epilepsy. Accessed November, 2021. 5. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy — United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821 - 825. 6. Epilepsy Foundation. What is Epilepsy? https://www.epilepsy.com/learn/about - epilepsy - basics/what - epilepsy. Accessed November, 2021. 7. Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active Epilepsy and Seizure Control in Adults — United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep 2018; 67:437 – 442. DOI: http://dx.doi.org/10.15585/mmwr.mm6715a1 • Epilepsy is a chronic disorder characterized by recurrent seizures 1 . • Epilepsy is defined as having two or more unprovoked seizures separated by at least 24 hours or after one seizure with a high risk of more 2 . • A seizure is a sudden surge of electrical activity in the brain caused by complex chemical changes that occur in nerve cells 3 . • Usually, there is a balance of cells that either encourage or stop other brain cells from sending messages 3 . • A seizure occurs when there may be too much or too little electrical activity in the brain causing an imbalance 3 . • Seizures are a symptom of many different disorders that can affect the brain 3 . Disease Overview Market Opportunity • Nearly 50 million people suffer from epilepsy worldwide, including 3 million adults and 470,000 children in the U.S 1,4,5 . • 150,000 new cases of epilepsy are reported in the United States each year 6 . • According to the CDC, 56% of adults living with diagnosed epilepsy continue to have seizures 7 .

49 JL: can will create a transition slide? IMPORTANT SAFETY INFORMATION

WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE - THREATENING RESPIRATORY DEPRESSION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CENTRAL NERVOUS SYSTEM (CNS) DEPRESSANTS Addiction, Abuse, and Misuse OLINVYK exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk before prescribing OLINVYK, and monitor all patients regularly for the development of behaviors or conditions. Life - Threatening Respiratory Depression Serious, life - threatening, or fatal respiratory depression may occur with use of OLINVYK. Monitor for respiratory depression, especially during initiation of OLINVYK or following a dose increase. Neonatal Opioid Withdrawal Syndrome Prolonged use of OLINVYK during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life - threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. Risk From Concomitant Use With Benzodiazepines or Other CNS Depressants Concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. Limitations of Use Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, reserve OLINVYK for use in patients for whom alternative treatment options [e.g., non - opioid analgesics or opioid combination products]: • Have not been tolerated, or are not expected to be tolerated • Have not provided adequate analgesia, or are not expected to provide adequate analgesia. The cumulative total daily dose should not exceed 27 mg, as total daily doses greater than 27 mg may increase the risk for QTc interval prolongation. CONTRAINDICATIONS OLINVYK is contraindicated in patients with: Significant respiratory depression Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment Known or suspected gastrointestinal obstruction, including paralytic ileus Known hypersensitivity to oliceridine (e.g., anaphylaxis) WARNINGS AND PRECAUTIONS OLINVYK contains oliceridine, a Schedule II controlled substance, that exposes users to the risks of addiction, abuse, and misuse. Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed OLINVYK. Assess risk, counsel, and monitor all patients receiving opioids. Serious, life - threatening respiratory depression has been reported with the use of opioids, even when used as recommended, especially in patients with chronic pulmonary disease, or in elderly, cachectic and debilitated patients. The risk is greatest during initiation of OLINVYK therapy, following a dose increase, or when used with other drugs that depress respiration. Proper dosing of OLINVYK is essential, especially when converting patients from another opioid product to avoid overdose. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status. Opioids can cause sleep - related breathing disorders including central sleep apnea (CSA) and sleep - related hypoxemia with risk increasing in a dose - dependent fashion. In patients who present with CSA, consider decreasing the dose of opioid using best practices for opioid taper. INDICATIONS AND USAGE OLINVYK is a new chemical entity indicated in adults for the management of acute pain severe enough to require an intravenous opioid analgesic and for whom alternative treatments are inadequate. 50

WARNINGS AND PRECAUTIONS Prolonged use of opioids during pregnancy can result in withdrawal in the neonate that may be life - threatening. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using OLINVYK for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. P r o f ound s e d a tion, re spi r a to r y d e p r e ssion, c om a , a nd d ea th m a y re sult f r om the c on c omit a nt use of OLINVYK with b e n z odi a z e pin e s or oth e r C NS d e p re ss a nts ( e . g ., non - b e n z odi a z e pine s e d a tiv e s/ h y pnoti c s, a n x i o l y ti c s, t ra nquili z er s, mus c le re l a x a nts, g e n e ra l a n e sth e ti c s, a ntip s y c hoti c s, oth e r opioids, or a l c ohol ) . B e ca u s e o f th e se r isks, re s er ve c o n c omit a nt p re s cr ibi n g of th e se d r u g s f o r use in p a ti e nts f or whom a lt er n a tive t rea tm e nt options a r e in a d e qu a t e , prescribe the lowest effective dose, and minimize the duration. OLINVYK was shown to have mild QTc interval prolongation in thorough QT studies where patients were dosed up to 27 mg. Total cumulative daily doses exceeding 27 mg per day were not studied and may increase the risk for QTc interval prolongation. Therefore, the cumulative total daily dose of OLINVYK should not exceed 27 mg. Increased plasma concentrations of OLINVYK may occur in patients with decreased Cytochrome P450 (CYP) 2D6 function or normal metabolizers taking moderate or strong CYP2D6 inhibitors ; also in patients taking a moderate or strong CYP3A4 inhibitor, in patients with decreased CYP2D6 function who are also receiving a moderate or strong CYP3A4 inhibitor, or with discontinuation of a CYP3A4 inducer. These patients may require less frequent dosing and should be closely monitored for respiratory depression and sedation at frequent intervals. Concomitant use of OLINVYK with CYP3A4 inducers or discontinuation of a moderate or strong CYP3A4 inhibitor can lower the expected concentration, which may decrease efficacy, and may require supplemental doses. Cases of adrenal insufficiency have been reported with opioid use (usually greater than one month). Presentation and symptoms may be nonspecific and include nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If confirmed, treat with physiologic replacement doses of corticosteroids and wean patient from the opioid. OLINVYK m a y ca u s e s e v e r e h y po t e ns i o n, i n c l u d i n g or t h o s tatic h y po t e ns i o n a n d s yn c op e in a m b u lat o r y p atie n t s . T h e r e is i n c r ea s ed r i s k in p ati e n ts w ho s e a b ili t y to m a i n ta i n b l oo d pr e ssu r e h as a l r ea d y b een c o m pr o m i s ed b y a r e d u ced b l oo d v o l u m e o r c o n c u rr e n t a d m i n i s t r ati o n o f c e r tain C N S d e pr e ss a n t dru g s ( e . g . , phenothiazines or g e n e r al a n e s t h eti c s ). M o n it o r t h e s e p atie n ts f o r s i g n s o f h y po t e ns i o n. I n p atie n t s w i t h ci r c u lat o r y s h o c k , avoid the use of OLINVYK as it m a y c a us e v a s od ilati o n t h at can f u r t h er r e d u ce ca rd iac o u t p u t a n d b l oo d pr e ssu r e. A v o id t h e us e o f OLINVYK in p atie n ts w i th i m p ai r ed c o ns ci o u s n e s s o r c o m a. OLINVYK should be used with caution in p atie n ts w h o m a y b e s us ce p ti b le to t h e i n t r ac r a n ial e f f ec t s o f C O 2 r et e n ti o n, such as t h o s e with e v i d e n ce o f i n c r ea s ed i n t r ac r a n ial pr e s su r e o r br ain t u m or s , as a reduction in r e s p i r at or y dr i v e a n d t h e r e su lt a n t C O 2 r et e n ti o n can f u r t h er i n c r ea s e i n t r ac r a n i al pr e ssu r e. Mo n it o r su c h p at i e n ts f o r s i g n s o f s e d ati o n a n d r e s p i r at or y d e pr e ss i o n , p a r tic u la r l y w h en i n i t iat i n g t h e r a p y. As with all opioids, OLINVYK m a y ca u s e s p a s m o f t h e s p h i n c ter o f O dd i , and m a y ca us e i n c r ea s es i n s e r u m a my la s e. Mo n it o r p atie n ts w i t h b ilia r y t r act d i s ea s e, i n c l u d i n g a c u te p a n c r eatit i s , f o r w o r s e n i n g s y m p t o ms . T h e r e is i n c r ea s ed r i s k in p ati e n ts w ho s e a b ili t y to m a i n ta i n b l oo d pr e ssu r e h as a l r ea d y b een c o m pr o m i s ed b y a r e d u ced b l oo d v o l u m e o r c o n c u rr e n t a d m i n i s t r ati o n o f c e r tain C N S d e pr e ss a n t dru g s ( e . g . , phenothiazines or g e n e r al a n e s t h eti c s ). M o n it o r t h e s e p atie n ts f o r s i g n s o f h y po t e ns i o n. I n p atie n t s w i t h ci r c u lat o r y s h o c k , avoid the use of OLINVYK as it m a y c a us e v a s od ilati o n t h at can f u r t h er r e d u ce ca rd iac o u t p u t a n d b l oo d pr e ssu r e. A v o id t h e us e o f OLINVYK in p atie n ts w i th i m p ai r ed c o ns ci o u s n e s s o r c o m a. OLINVYK should be used with caution in p atie n ts w h o m a y b e s us ce p ti b le to t h e i n t r ac r a n ial e f f ec t s o f C O 2 r et e n ti o n, such as t h o s e with e v i d e n ce o f i n c r ea s ed i n t r ac r a n ial pr e s su r e o r br ain t u m or s , as a reduction in r e s p i r at or y dr i v e a n d t h e r e su lt a n t C O 2 r et e n ti o n can f u r t h er i n c r ea s e i n t r ac r a n i al pr e ssu r e. Mo n it o r su c h p at i e n ts f o r s i g n s o f s e d ati o n a n d r e s p i r at or y d e pr e ss i o n , p a r tic u la r l y w h en i n i t iat i n g t h e r a p y. As with all opioids, OLINVYK m a y ca u s e s p a s m o f t h e s p h i n c ter o f O dd i , and m a y ca us e i n c r ea s es i n s e r u m a my la s e. Mo n it o r p atie n ts w i t h b ilia r y t r act d i s ea s e, i n c l u d i n g a c u te p a n c r eatit i s , f o r w o r s e n i n g s y m p t o ms . OLINVYK m a y i n c r ea s e t h e f r e q u e n c y o f s ei z u r es i n p atie n t s w i t h s eiz u r e d i s ord e r s a n d may increase t h e r i s k o f s ei z u r es in vulnerable patients . M o n it o r p atie n ts w i th a h i s t o r y o f s ei z u r e d i s ord e r s f o r w or s e n ed s eiz u r e c o n t ro l. Do not abruptly discontinue OLINVYK in a patient physically dependent on opioids. Gradually taper the dosage to avoid a withdrawal syndrome and return of pain. Avoid the use of mixed agonist/antagonist (e.g., pentazocine , nalbuphine , and butorphanol ) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving OLINVYK, as they may reduce the analgesic effect and/or precipitate withdrawal symptoms. OLINVYK may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Although self - administration of opioids by patient - controlled analgesia (PCA) may allow each patient to individually titrate to an acceptable level of analgesia, PCA administration has resulted in adverse outcomes and episodes of respiratory depression. Health care providers and family members monitoring patients receiving PCA analgesia should be instructed in the need for appropriate monitoring for excessive sedation, respiratory depression, or other adverse effects of opioid medications. ADVERSE REACTIONS Adverse reactions are described in greater detail in the Prescribing Information. The most common (incidence ≥10%) adverse reactions in Phase 3 controlled clinical trials were nausea, vomiting, dizziness, headache, constipation, pruritus, and hypoxia. PLEASE see www.OLNVYK.com for full prescribing information including BOXED warning and important safety information 51